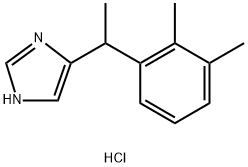
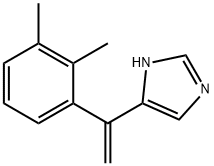
Description
Medetomidine hydrochloride is a potent, highly selective α2-adrenoceptor agonist (Ki values are 1.08 and 1750 nM for α2- and α1-adrenoceptors respectively). It is an imidazole compound. Medetomidine hydrochloride shows greater selectivity over α1-adrenoceptors than clonidine and UK 14,304 (1620-, 220- and 300-fold respectively). In addition, it influences the adrenergic system by mimicking the action of adrenergic neurotransmitters.
Chemical Properties
White Solid
Uses
An α2-Adrenergic agonist. Sedative; analgesic.
Uses
Medetomidine is a selective α2-adrenoceptor agonist, with Ki of 1.08 nM, exhibts 1620-fold selectivity over α1-adrenoceptor
Definition
ChEBI: Medetomidine hydrochloride is a hydrochloride.
Indications
Medetomidine hydrochloride is an FDA-approved alpha2-adrenoceptor (α2-AR) agonist with analgesic, sedative and anxiolytic properties for use as a veterinary sedative. It has been approved for use in animals such as cats, sheep, and dogs (12 weeks of age and older.) In 1999, the FDA approved Medetomidine for human use in the sedation of intubated and mechanically ventilated intensive care unit (ICU) patients, including use as a pain reliever and for use in non-intubated patients prior to and during surgery and other procedures. In addition to its pharmaceutical uses, it is used as an antifouling agent in marine coatings.
[1]
Biological Activity
medetomidine is a selective α2-adrenoceptor agonist, with ki of 1.08 nm, exhibts 1620-fold selectivity over α1-adrenoceptor.
Pharmacokinetics
After intramuscular injection, medetomidine is rapidly and almost completely absorbed at the site of injection and its pharmacokinetics are very similar to that observed after intravenous injection. Maximum plasma concentrations are reached within 15 to 20 minutes. Estimated plasma half-life is 1.2 hours for dogs and 1.5 hours for cats. Medetomidine is mainly oxidised in the liver, while a small amount is methylated in the kidney. Metabolites are primarily excreted in urine.
Side effects
Medetomidine hydrochloride is an alpha-2 adrenoreceptor agonist. Symptoms after absorption may involve clinical effects, including dose dependent sedation, respiratory depression, bradycardia, hypotension, a dry mouth, and hyperglycaemia. Ventricular arrhythmias have also been reported. Respiratory and haemodynamic symptoms should be treated symptomatically.
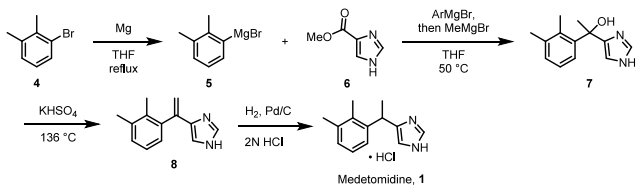
Synthesis
Medetomidine hydrochloride is synthesized using 2,3-Dimethylbromobenzene as the starting material through a series of Grignard reactions. This process yields tertiary alcohol 7, which is subsequently subjected to dehydration and hydrogenation. The overall yield achieved was 17 percent.
[1] The reaction scheme is illustrated as follows:
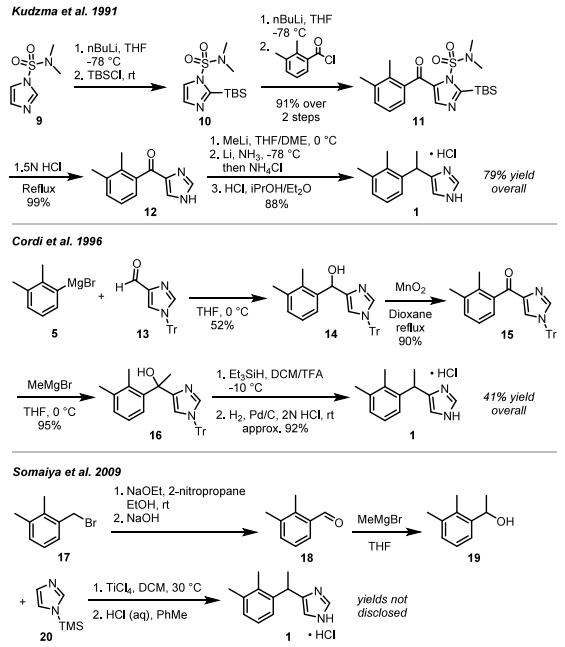
Additional synthetic processes encompass:
References
[1] PEDRO DE ANDRADE HORN. Classics in Chemical Neuroscience: Medetomidine.[J]. ACS Chemical Neuroscience, 2024: 3874-3883. DOI:10.1021/acschemneuro.4c00583.