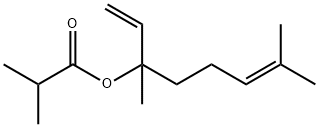
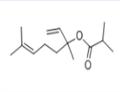
Description
Linalyl isobutyrate has a light, fruity odor with a lavender note
and a sweet flavor reminiscent of black currant. May be synthe sized by esterification of linalool with isobutyric anhydride.
Chemical Properties
Linalyl Isobutyrate has a fresh, fruity
lavender odor,which is more refined than that of the butyrate. It is used in lavender
compositions and in several floral notes.
Occurrence
Reported found in the essential oil of Ceylon cinnamon and in lavender oil; a dextrorotatory form has been
reported in the oil from leaves of Agathosoma gnidioides and sour cherry.
Uses
Linalyl Isobutyrate is a flavoring agent that is a liquid, slightly
yellow in color with a fruity odor. it is miscible in alcohol, ether,
and chloroform, and insoluble in water. it is obtained by chemical
synthesis. it is also termed 3,7-dimethyl-2,6-octadien-3-yl isobutrate.
Definition
ChEBI: Linalyl isobutyrate is a monoterpenoid.
Application
Linalyl Isobutyrate is used in perfume compositions, mainly in Lavender complexes, Bergamot bases, Lilac and Citrus-colognes, etc. The ester is also used in Banana, Blackcurrant, Cherry, Pear, Pineapple, Plum and other imitation fruit flavours, as well as in Nut, Spice, Citrus, Berry and various fruit complexes and bases. The concentration varies from 2 to 15 ppm in the finished product.
Preparation
By esterification of linalool with isobutyric anhydride.
Taste threshold values
Taste characteristics at 20 ppm: floral, fruity, sweet, berry and citrus.
Toxicity evaluation
In the rat, the acute oral LD 50
was reported to be > 36.3 g/kg (Jenner, Hagan,
Taylor, Cook & Fitzhugh, 1964). At this dose, the highest tested, toxic signs included depression,
wet fur and diarrhoea, but the surviving animals appeared normal after 1 wk. In the mouse, the
acute oral LD 50
was 151 g/kg (Jenner et al. 1964); animals were depressed soon after treatment
and excitable after 1 hr, with rough fur, and death occurred between 4 hr and 3 days. The acute
dermal LD 50
in rabbits was reported as > 5 g/kg (Moreno, 1974).