Originator
Nanbacine,Fournier,France,1976
Definition
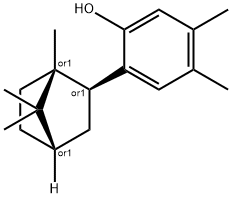
ChEBI: A bridged compound that is 3,4-xylenol carrying an additional isobornyl substituent at position 6. A lipophilic antibacterial drug mainly used in spray dosage forms for the local treatment of infection and inflammation of the throat.
Manufacturing Process
100 g of 3,4-xylenol and 150 g of camphene are melted in a two-necked flask
equipped with a reflux condenser and a thermometer. 10 g of stannic chloride
are added in small quantities; the temperature is kept between 70°C and
80°C for 4 hours. The mass is then allowed to cool and 300 ml of benzene
and 300 ml of water are added. The aqueous layer is decanted off, and the
supernatant organic layer is washed, first with 1,200 ml of 10% potassium
hydroxide and then with water until neutral. The benzene is driven off and the
mass is distilled. The fraction which passes between 203°C and 223°C/200
mm Hg is collected and recrystallized in petroleum ether.
100 mg of the recrystallized product is dissolved in 10 ml of hexane.
This solution is then slowly passed through a chromatographic alumina
column, 20 cm in length and 16 mm in diameter, containing 20 g of alumina
(Prolabo).
The column is then eluted with benzene and 2 ml fractions of the eluent are
collected as soon as the product appears in the eluent. The presence of the
product is detected by means of the color change in the collected eluent after
adding 1 drop of 2% ferric chloride and 2 drops of 5% potassium ferricyanide
solution.
18 ml of a first fraction are collected, the next 2 ml of eluent are discarded
and then a second fraction of 20 ml is collected. Removal of the solvent from
the first fraction by distillation leaves a product having a melting point of
between 94°C and 96°C and removal of the solvent from the second fraction
leaves a product having a melting point between 86°C and 88°C.
The product remaining from the first fraction is 6-isobornyl-3,4-xylenol while
that from the second fraction is its isomer 6-exo-isocamphenyl-3,4-xylenol.