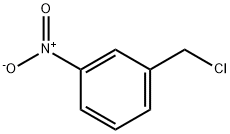
Chemical Properties
yellow to brown crystalline solid
Uses
3-Nitrobenzyl chloride was used in the synthesis of 8-chloropurine.
General Description
Yellow to brown solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A halogenated aromatic nitro compound. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.
Fire Hazard
Flash point data concerning 3-Nitrobenzyl chloride are not available, however, 3-Nitrobenzyl chloride is probably combustible.
Purification Methods
Crystallise the chloride from pet ether (b 90-120o). IRRITANT.[Beilstein 5 IV 855.]