Description
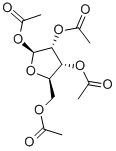
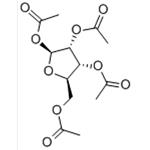
beta-D-Ribofuranose 1,2,3,5-tetraacetate is an acetylated derivative of ribose extensively used in carbohydrate research and synthetic chemistry. This compound features acetyl groups protecting the hydroxyl functionalities at positions 1, 2, 3, and 5 of the ribofuranose ring, which enhances its stability and solubility in organic solvents. β-D-Ribofuranose 1,2,3,5-tetraacetate is a crucial intermediate in synthesising nucleosides, nucleotides, and other complex carbohydrates. In research, it is utilized to study glycosylation mechanisms, focusing on the formation and stereochemistry of glycosidic bonds.
Chemical Properties
beta-D-Ribofuranose 1,2,3,5-tetraacetate is white to almost white crystalline powder
Uses
beta-D-Ribofuranose 1,2,3,5-tetraacetate is used in the synthesis of 3-(β-D-ribofuranosyl)-2,3-dihydro-6H-1,3-oxazine-2,6-dione, a new pyrimidine nucleoside analog related to uridine.
Application
beta-D-Ribofuranose 1,2,3,5-tetraacetate, also known as 1,2,3,5-tetra-O-Acetyl-beta-D-ribofuranose (TAR), is an important organic reagent that can be used in the following pathways:
(1) Used in low-energy electron-induced reactions in gas-phase TAR. Since the acetyl group in TAR is coupled to the five-membered ribose ring at relevant positions, it can serve as a suitable model compound for studying the reaction of sugar units in DNA to low-energy electrons[1].
(2) Used to prepare 1,2,3-tri-O-acetyl-β-d-ribofuranose by enzymatic deacetylation[2].
(3) Used in condensation reactions. In the presence of tin chloride, 1-hexene reacts with 1- O -acetyl-2,3,5-tri- O -benzoyl-β- d -ribofuranose ( 5b ) or 1,2,3,5-tetra- O -acetyl-β- d -ribofuranose ( 5a ) to form a complex mixture[3].
Synthesis
Take 1000mL flask, add 50g of inosine, acetic anhydride 200ml heating to reflux with stirring, the temperature of the inner liquid 120, about 60 minutes clarification, reduce the temperature to 100, add the catalyst (NaSiO3 and Na2HPO4 according to the 2:1 mixing) 10g at 110 holding time of 2h, connected to the Wiegler fractional distillation column, in the micro-negative pressure, evaporate away from the acetic acid and acetic anhydride mixture, evaporate away from the acid 100ml, about 100 minutes. The mixture of acetic acid and acetic anhydride was evaporated under slightly negative pressure, 100 ml of acid was evaporated for about 100 min, and it was kept at 110C for 6 h. The end point was tracked with a thin-layer chromatographic plate until the basic disappearance of triacetylinosine. Cool down to 30 , filtration, combined filtrate, in the rotary evaporator will be mixed acetic anhydride vacuum evaporation, the residue will be cold to 90-100 , add distilled water, 150 ml, stirring under the crystallization, precipitation of tetraacetyl ribose, which yield is 94.8%.
References
[1] ILKO BALD. Low energy electron-induced reactions in gas phase 1,2,3,5-tetra-O-acetyl-beta-D-ribofuranose: a model system for the behavior of sugar in DNA.[J]. Journal of Chemical Physics, 2007. DOI:10.1063/1.2436873.
[2] TUN-CHENG CHIEN; Ji W C. A convenient preparation of 1,2,3-tri-O-acetyl-β-d-ribofuranose by enzymatic regioselective 5-O-deacetylation of the peracetylated ribofuranose[J]. Carbohydrate Research, 2004. DOI:10.1016/j.carres.2004.01.010.
[3] THOMAS L. CUPPS; Leroy B T; Dean S Wise. A further investigation of the stannic chloride-catalyzed condensation reaction of 1-hexene and 1,2,3,5-tetra-O-acyl-β-d-ribofuranoses[J]. Carbohydrate Research, 1983. DOI:10.1016/0008-6215(83)88135-X.