Description
Gefapixant was approved in Japan in 2022 for adult patients with refractory or unexplained chronic cough, marketed under the name Lyfnua.The drug was authorised by the European Medicines Agency (EMA) in September 2023 for the same indication. However, the FDA refused to approve gefapixant for the treatment of adults with refractory or unexplained chronic cough.
Uses
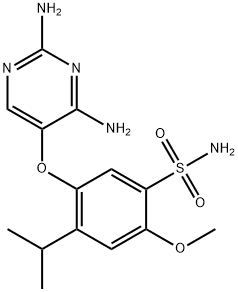
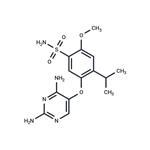
Gefapixant is an orally active and potent purinergic P2X3 receptor (P2X3R) antagonist, with IC50 values of ~30 nM versus recombinant hP2X3 homotrimers and 100-250 nM at hP2X2/3 heterotrimeric receptors. Gefapixant can be used for the research of chronic cough and knee osteoarthritis.