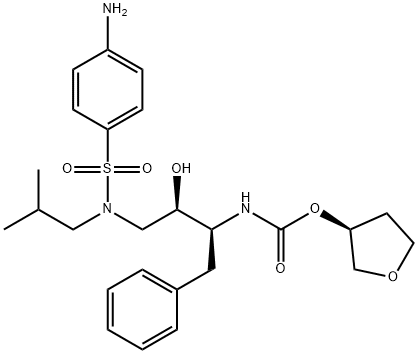
Description
Amprenavir was launched as Agenerase in the US for the treatment of
AIDS patients in combination with approved agent antiretroviral nucleoside
analogs. It is the fifth non-peptidic inhibitor of HIV-1 protease to be marketed in
this indication after the last approved Neflinavir. Amprenavir, designed via a
structure-based process, is the smallest molecule in the 《navir》 class and
exhibits a reduced peptidic character. An improved process for preparation
comprising four steps from a (1S, 2R)-2-hydroxy-3-aminopropylcarbamate has
been developed. Amprenavir is a potent inhibitor of HIV-1 aspartyl protease (Ki
= 0.6nM), an enzyme required by the virus to cleave pro-form polyproteins to
structural proteins during the last stage in the replication process. The
compound displays good oral bioavailability in humans and penetrates the CNS,
which is an important advantage in long-term treatment. Its plasma half-life is
approximately 10h. Treatment with Amprenavir in combination with nucleoside
analog reverse transcriptase inhibitors considerably decreases viral load and
restores CD4+ T-cell counts in patients with HIV infection.
Description
Amprenavir is an inhibitor of HIV protease (K
i = 0.04 nM). It inhibits the cytopathic effects of HIV-1 in MT-4 cells (IC
50 = 150 nM). Formulations containing amprenavir have been used in combination with other antiretroviral agents in the treatment of HIV-1 infection.
Chemical Properties
Off-White to Pale Yellow
Originator
Vertex Pharm (US)
Uses
Protease inhibitor, anti-HIV agent
Uses
A selective HIV protease inhibitor. An analogue of Ritonavir
Indications
Amprenavir (Agenerase) is administered twice daily,
providing the patient with an advantage over other protease
inhibitors that must be taken more frequently
(e.g., indinavir, saquinavir). Common side effects of am-prenavir include nausea, vomiting, diarrhea, and perioral
paraesthesias. Rash occurs in approximately 20 to
30% of patients and can be mild or severe (Stevens-
Johnson syndrome).
Definition
ChEBI: Amprenavir is a tetrahydrofuryl ester, a sulfonamide and a carbamate ester. It has a role as a HIV protease inhibitor and an antiviral drug.
Manufacturing Process
(1-Oxiranyl-2-phenylethyl)-carbamic acid t-butyl ester may be synthesized
from available starting materials (see B. E. Evans et al., J. Org. Chem., 50,
p.4615 (1985)). The amprenavir may be preparated with the next steps.
1. (1-Benzyl-3-isobutylaminopropyl)-carbamic acid t-butylester. A solution of
4.1 g of epoxide (1-oxiranyl-2-phenylethyl)-carbamic acid t-butyl ester in 30
ml of ethanol was treated with 22.4 ml of isobutylamine and heated under
reflux for 1 h. The mixture was concentrated to yield the title compound as a
white solid which was used without subsequent purification.
2. To a solution of the above compound (2.5 g, 7.43 mmol) in CH2Cl2 (50 ml)
was added triethylamine (2.1 ml, 14.9 mmol) followed by addition of benzyl
chloroformate (1.2 ml, 8.1 mmol). The mixture was allowed to stir at ambient
temperature for 6 h. The solution was diluted with 1 L of CH2Cl2 and washed
with water. The organics were dried over anhydrous MgSO4, concentrated under reduced pressure, then purified via silica gel chromatography. Gradient
solvent system: CH2Cl2 followed by 3:97 methanol/CH2Cl2. (3-t-
Butoxycabonylamino-4-phenylbutyl)-isobutylcarbamic acid benzyl ester (2.97
g) was obtained as a colorless oil. TLC: Rf= 0.14, 3:97 methanol/CH2Cl2.
3. BOC - protecting group was removed as followed: to a solution of 1.5 g
(3.187 mmol) of the above compound of in ethyl acetate (25 ml) at - 20°C
was bubbled anhydrous HCl gas for 10 min. The ice bath was removed and
after an additional 15 min the reaction mixture was sparged with nitrogen,
then concentrated in vacuo to provide 1.29 g of deprotected product as a
white solid which was used directly for the next reaction TLC: Rf= 0.14, 10%
methanol/CH2Cl2.
4. Isobutyl[4-phenyl-(S)-3-tetrahydrofuran-3-yl-oxycarbonylamino)-butyl]-
carbamic acid benzyl ester. To a solution of 1.077 g of the above resultant
crude compound (2.647 mmol) in acetonitrile (10 ml) was added sequentially
at ambient temperature under an atmosphere of nitrogen, 1.61 ml (9.263
mmol) of diisopropylethylamine and 910 mg (3.97 mmol) of the Nsuccinimidyl-(
S)-3-tetrahydrocfuryl carbonate. The last one was prepared from
phosgene and (S)-(+)-3-hydroxytetrahydrofuran by usual procedure. After
stirring for 3 h, an additional 223 mg (0.973 mmol) of the N-succinimidyl-(S)-
3-tetrahydrocfuryl carbonate was added. The mixture was stirred for 16 h and
then concentrated in vacuo. The residue was taken up in ethyl acetate and
washed with water, 0.5 N HCl, saturated sodium bicarbonate, saturated brine,
dried over magnesium sulfate, filtered, and concentrated in vacuo. The
residue was purified by low pressure silica gel column chromatography using a
gradient 10% to 25% ethyl acetate in CH2Cl2 eluent to yield 1.025 g of the
title product as a white solid. TLC: Rf= 0.10, 10% ethyl acetate/CH2Cl2.
5. A solution of 872 mg (1.799 mmol) isobutyl[4-phenyl-(S)-3-
tetrahydrofuran-3-yl-oxycarbonylamino)-butyl]-carbamic acid benzyl ester in
(10 ml) of ethyl alcohol was added, at ambient temperature under a nitrogen
atmosphere, to a slurry of 87 mg (10% by weight) of 10% palladium on
carbon in (5 ml) ethyl alcohol and hydrogenated for 16 h under a slight
positive pressure of hydrogen. The mixture was filtered and concentrated in
vacuo to yield 553.2 mg of the carbamic acid as a colorless glass which was
used directly for ensuing reaction. TLC: Rf = 0.46, 10% methanol/CH2Cl2.
6. A solution of 102 mg of the resultant compound of a step 5 in 4:1
CH2Cl2/saturated aqueous NaHCO3 was treated sequentially, at ambient
temperature under an atmosphere of nitrogen, with 65 mg of pnitrobenzenesulfonyl
chloride and 51 mg of sodium bicarbonate. The mixture
was stirred for 14 h, diluted with CH2Cl2, washed with saturated NaCl, then
dried over MgSO4, filtered, and concentrated in vacuo. The residue was
purified by low pressure silica gel chromatography using 20% diethyl
ether/CH2Cl2 as eluent to provide 124 mg of the 1-benzyl-3-[isobutyl-(4-
nitrobenzenesylfonyl)-amino]-propylcarbamic acid tetrahydrofuran-3(S)-yl]
ester as a white solid. TLC: Rf = 0.36, 20% diethyl ether/CH2Cl2, HPLC: Rt =
15.15 min. (1H)-NMR (CDCl3) consist with structure.
7. A solution of 124 mg of the resultant compound of the step 6 in ethyl
acetate was treated, at ambient temperature, with 13 mg of 10% palladium on carbon. The mixture was stirred for 14 h under an atmosphere of
hydrogen, filtered through a pad of celite filter agent, and concentrated in
vacuo. The residue was subjected to preparative HPLC to yield 82 mg of the
((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-
(phenylmethyl)propyl)-, (3S)-tetrahydro-3-furanyl carbamic ester a white
solid. TLC: Rf= 0.10, 20% ether/CH2Cl2, HPLC: Rt= 13.16 min. (1H)-NMR
(CDCl3) consistent with structure.
brand name
Agenerase
(GlaxoSmithKline).
Therapeutic Function
Antiviral
Acquired resistance
Mutations at position 50, 76 and 84 of the protease enzyme
gene are associated with significantly reduced susceptibility.
General Description
Amprenavir is a second-generation drug derived from hydroxyethylamine sulfonamide.
Pharmaceutical Applications
A synthetic compound formulated as the calcium salt of the
oral prodrug fosamprenavir.
Biochem/physiol Actions
Protease inhibition results in inactive and immature virus.
Pharmacokinetics
Oral absorption: Not known/available
C
max 700 mg + ritonavir 100 mg:c. 6.08 mg/L
twice daily
C
min 700 mg + ritonavir 100 mg:c. 2.12 mg/L
twice daily
Plasma half-life: c. 7.7 h
Volume of distribution: c. 430 L
Plasma protein binding: c. 90%
Absorption
Fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate by cellular phosphatases in the gut epithelium as it is absorbed. Absolute bioavailability has not been established. It can be taken without regard to food.
Distribution
It penetrates moderately well into the CNS. The semen:plasma ratio is negligible. It is not known if it is distributed into breast milk.
Metabolism and excretion
It is extensively metabolized by the cytochrome P
450 (CYP) 3A4 enzyme system. Two major metabolites have been identified that appear to result from the oxidation of the tetrahydrofuran and aniline moieties. Around 14% of a dose is eliminated in the urine and 75% in feces, <3% as unchanged drug. Metabolites account for >90% of administered drug found in fecal samples. It should be used with caution and at reduced doses in adults with mild or moderate hepatic impairment; it is contraindicated in patients with severe hepatic impairment.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Side effects
The most common adverse events in patients receiving boosted
fosamprenavir were diarrhea, nausea, headache, fatigue,
vomiting
and rash. Ritonavir-boosted fosamprenavir is associated
with a dyslipidemia profile characteristic of those
treated with other protease inhibitors boosted with 200 mg
of ritonavir.