Originator
Naftidan,De Angeli,Italy,1969
Manufacturing Process
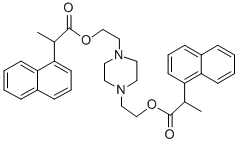
15 grams of α-methyl-1-naphthylacetic acid were refluxed with 50 ml of
thionyl chloride during 3 hours. The excess thionyl chloride was removed
under reduced pressure and the product was also isolated by distillation under
reduced pressure. Yield: 15.6 grams (96%). The α-methyl-1-naphthyl acetyl
chloride boils at 120° to 124°C. 1.76 grams of N,N'-di-(β-hydroxyethyl)-
piperazine, 1.9 grams of sodium bicarbonate and 4.45 grams of α-methyl-1-
naphthyl acetyl chloride in 30 ml of anhydrous acetonitrile were refluxed with
stirring during 5 hours. After cooling the mixture was filtered and the acetonitrile evaporated off under reduced pressure. 5.2 grams of crude ester
were obtained. The hydrochloride, melting at 220° to 221°C, may be prepared
by dissolving the ester in absolute ethanol and treating the solution with
anhydrous gaseous hydrogen chloride.