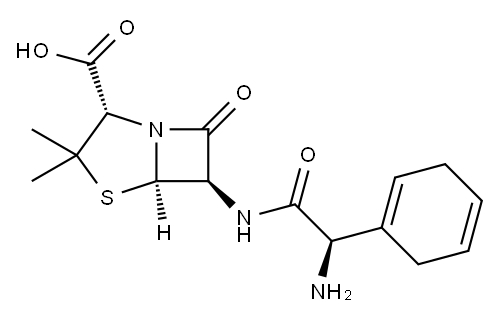
Originator
Dexacilline,Squibb,France,1974
Manufacturing Process
See Cephradine for preparation of D-2-amino-2-(1,4-cyclohexadienyl)acetic
acid and then its methyl acetoacetic ester enamine as the starting material.
358 mg of 6-aminopenicillanic acid (APA) (1.66 mmol) are stirred well in 2.5
ml of water while 0.23 ml triethylamine is gradually added with the pH kept
under 8.0. Final pH is 7.4; 0.85 ml acetone is added and the solution kept at -
10°C.
469 mg methyl acetoacetate enamine of D-2-amino-2-(1,4-
cyclohexadienyl)acetic acid sodium salt (1.715 mmol) are stirred in 4.25 ml
acetone at -20°C. A microdrop of N-methylmorpholine is added followed by
the slow addition of 198 mg of ice cold ethyl chloroformate. Water, 0.43 ml, is
added at this point and a turbid solution results. The reaction mixture is
stirred for 10 minutes at -20°C.
The turbid solution of mixed anhydride is then added to the 6-APA solution. A
complete solution is observed. The solution is stirred for 30 minutes at -10°C,
then raised to room temperature, acidified to pH 2.0 with diluted HCl and,
with good stirring, the pH is kept at that level for 10 minutes.
The solution is then extracted with 5 ml xylene. The aqueous layer is layered
with 5 ml methyl isobutyl ketone and the pH adjusted to 5.0 with 1 N NaOH
and chilled overnight. The resulting crystals are filtered off, washed with water
and air dried. Yield, 272 mg (44%), decomposes at 202°C.
Antimicrobial activity
An analog of ampicillin in which the benzene ring is partially
saturated. It closely resembles ampicillin in its antibacterial
properties. It is moderately well absorbed, a 500 mg oral dose
producing mean peak plasma levels of 2–3 mg/L. Its behavior
on intramuscular injection, distribution, excretion, toxicity
and uses resemble those of ampicillin. It is of very limited
availability.