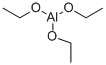
Chemical Properties
liquid that slowly solidifies to a white powder(s); sensitive to moisture; prepared from a reaction of Al with ethanol in the presence of catalytic amounts of I2 and HgCl2; used as a polymerization catalyst and to reduce aldehydes and ketones [MER06] [STR93] [HAW93]
Uses
Aluminum ethoxide is mainly used in industrial settings. It is used as a reducing agent for aldehyde, ketones, polymerization catalyst. Used in the sol-gel process preparation of high purity aluminum sesquioxide. It also used as a reducing reagent, for carbonyl compounds that restore to alcohol.
Uses
In the reduction of aldehydes and ketones; as catalyst for polymerizations.
Flammability and Explosibility
Flammable
reaction suitability
core: aluminum
Purification Methods
Crystallise it from CS2 [m 139o, CS2 complex] and distil it in a vacuum. The molecular weight corresponds to [Al(OEt)3]4 [Robinson & Peak J Phys Chem 39 1127 1935, Vilani & Nord J Am Chem Soc 69 2605 1947]. [Beilstein 1 H 313, 1 I 158, 1 II 3008, 1 III 1284, 1 IV 1289.]