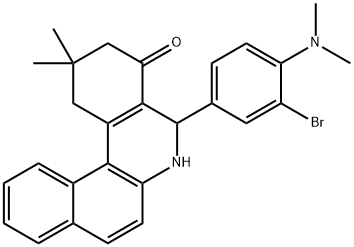
Description
GAC Inhibitor 968 (311795-38-7) is an allosteric inhibitor of glutaminase C (GAC).1 Inhibition of GAC blocks oncogenic transformation induced by Rho GTPases in fibroblasts and B lymphoma cells with no effect on normal cells.2 Inhibits liver-type glutaminase GLS2 and suppresses breast tumor growth in vivo.3,4 Reverses acquired erlotinib resistance in non-small cell lung cancer.5
Uses
Glutaminase inhibitor 968 has been used to study the role of glutamine metabolism in macrophage activation.
Definition
ChEBI: 5-[3-bromo-4-(dimethylamino)phenyl]-2,2-dimethyl-2,3,5,6-tetrahydrobenzo[a]phenanthridin-4(1H)-one is a partially hydrogenated benzophenanthridine carrying an oxo group at C-4, geminal methyl groups at C-2 and a 3-bromo-4-(dimethylamino)phenyl group at C-5. It has a role as an EC 3.5.1.2 (glutaminase) inhibitor.
General Description
Glutaminase Inhibitor 968 belongs to the benzophenanthridinone family.
Biochem/physiol Actions
Glutaminase Inhibitor 968 is an allosteric inihibitor of the mitochondrial enzyme glutaminase C (GAC), which is overexpressed in a number of cancer cell lines. Glutaminase Inhibitor 968 shows 21% inhibition at 10 μM, 94% at 25 μM. Glutaminase Inhibitor 968 blocked human cancer cell proliferation in culture, and in inhibited tumor formation in mouse xenograft models.
References
Stainecker et al. (2015), Mechanism by which a recently discovered allosteric inhibitor blocks glutamine metabolism in transformed cells; Proc. Natl. Acad. Sci. USA, 112 394
Wang et al. (2010), Targeting mitochondrial glutaminase activity inhibits oncogenic transformation; Cancer Cell, 18 207
Katt et al. (2012), Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation; Cancer Ther., 11 1269
Lukey et al. (2019), Liver-Type Glutaminase GLS2 Is a Druggable Metabolic Node in Luminal-Subtype Breast Cancer; Cell Reports, 29 76
Xie et al. (2016), Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer; Oncotarget, 7 610