Uses
Brightening groundwood, kraft, and other paper
pulps; to treat beet and cane sugar juices; depressant
in mining flotations; bleaching textiles, vegetable
oils, straw, hemp, vegetable tannins, animal glue,
etc.
General Description
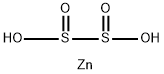
ZINC HYDROSULFITE is a white powder. ZINC HYDROSULFITE is noncombustible. ZINC HYDROSULFITE is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. ZINC HYDROSULFITE is used to bleach wood pulp, textiles, and other naturally occurring materials.
Air & Water Reactions
Water soluble. Mixing with limited quantities of water can cause heating and decomposition with release of irritating gaseous sulfur dioxide.
Reactivity Profile
A strong reducing agent. Inorganic reducing agents, such as ZINC HYDROSULFITE, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Their reactions with oxidizing agents may be violent. Sulfites and hydrosulfites (dithionites) can react explosively with strong oxidizing agents. Sulfites generate gaseous sulfur dioxide in contact with oxidizing acids and nonoxidizing acids.
Health Hazard
INHALATION: Irritation of nose and throat. EYES: Mild irritant. SKIN: Irritant. INGESTION: Nausea and vomiting.
Fire Hazard
Special Hazards of Combustion Products: Decomposes giving off irritating SO 2
Safety Profile
Probably a poison.
When heated to decomposition it emits
toxic vapors of zinc and SOx.