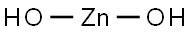Description
Zinc hydroxide is a white solid that usually forms in solution. It is not very stable as a dried solid, therefore a standard of zinc hydroxide is not available commercially.
Zinc hydroxide Zn(OH)2 is an amphoteric chemical compound. Both bases and acids react with zinc hydroxide. It's an insoluble hydroxide that dissolves when exposed to a strong acid. It occurs naturally as the rare minerals Wulfingite, ashoverite and sweetite. Zinc hydroxide is used as a biological absorbant often.
Chemical Properties
Colorless crystals.Decomposes
at 125C. Almost insoluble in water; forms
both zinc salts and zincates.
Physical properties
Colorless orthorhombic crystals; density 3.053 g/cm
3; decomposes at 125°C; slightly soluble in water.
Uses
Intermediate, absorbent in surgical dressings,
rubber compounding.
Preparation
The compound is prepared by adding a strong alkali to a solution of zinc sulfate or chloride: ZnSO
4 + 2NaOH → Zn(OH)
2 + Na
2SO
4.