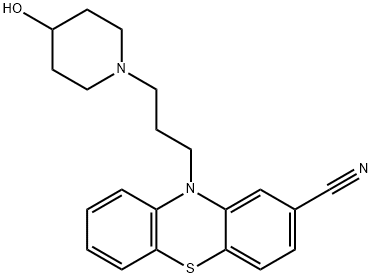
Chemical Properties
Yellow Solid
Originator
Aolept, Bayer Vital
Uses
Psychotherapeutic phenothiazine. Antipsychotic.
Uses
Spectrophotometric reagent for palladium and ruthenium.
Definition
ChEBI: A member of the class of phenothiazines that is 10H-phenothiazine substituted by a 3-(4-hydroxypiperidin-1-yl)propyl group at the nitrogen atom and a carbonitrile group at position 2. Periciazine is a first generation antipsychotic.
Manufacturing Process
2-Cyano-10-(3-methanesulfonyloxypropyl)phenthiazine and 4hydroxypiperidine in toluene were heated under reflux with stirring. The reaction mixture was allowed to cool and water was added. The resulting toluene solution layer was decanted and washed twice with water. The toluene solution was then stirred with 5% hydrochloric acid. The hydrochloride of the desired phenthiazine base precipitated in gummy condition in the aqueous layer. This was decanted and treated with sodium hydroxide (density 1.33). It was then extracted three times with ethyl acetate. The extracts were dried over sodium sulfate, filtered and concentrated in vacuum. A resinous product was obtained. This product was dissolved in a mixture of benzene and cyclohexane and chromatographed on a column containing alumina. The chromatographed product was eluted successively with mixtures of benzene and cyclohexane and then with benzene and finally with a mixture of benzene and ethyl acetate. The eluates were evaporated to yield a crude product. This product was recrystallised from aqueous ethanol (40% water) and yielded 2cyano-10-[3-(4-hydroxy-1-piperidyl)propyl]phenthiazine as white crystals.
Therapeutic Function
Neuroleptic
Safety Profile
Poison by ingestion,
intraperitoneal, intravenous, and
subcutaneous routes. Used as an
antipsychotic agent. When heated to
decomposition it emits very toxic fumes of
CN-, NOx, and SOx. See also NITRILES.
Purification Methods
It recrystallises from a saturated solution in cyclohexane. It is antipsychotic and is a sensitive reagent for Pd, Va, Ru, Rh and Au. [Gowda et al. Anal Chem 55 1816 1983, Anal Chim Acta 154 347 1983, Beilstein 27 III/IV 4110.]