Description
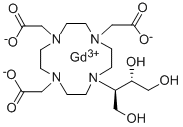
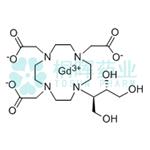
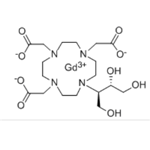
Gadobutrol is a nonionic, paramagnetic contrast agent developed for tissue contrast enhancement in magnetic resonance imaging (MRI). It has a macrocyclic framework and is neutral. It is a water-soluble, highly hydrophilic compound with a partition coefficient between n-butanol and buffer at pH 7.6 of ~ 0.006.
Uses
Gadobutrol is a gadolinium-based MRI contrast agent (GBCA).
Uses
Gadobutrol injection is a magnetic resonance imaging (MRI) contrast agent that is used to help create a clear picture of the body during an MRI scan. MRI scans are a special kind of procedure that lets a doctor look at the inside of the body, such as the brain.
brand name
Gadobutrol is marketed by Bayer AG as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist. In India, it is also marketed by Vivere Imaging as Viv-butrol.
Mechanism of action
In the central nervous system, Gadobutrol works by highlighting any areas with disrupted blood brain barrier (BBB) and/or abnormal vascularity. In breast tissue, Gadobutrol exposes the presence and extent of malignant breast disease.
Side effects
Side effects of Gadobutrol are uncommon but may include: headache,
nausea,
vomiting,
feeling unwell (malaise),
dizziness,
abnormal or unpleasant taste in your mouth,
feeling hot,
numbness or tingly feeling,
itching or rash,
skin redness, or
injection site reactions (cold feeling, warmth, pain, or burning). Its more serious side effects include: urinating less than usual or not at all; drowsiness, confusion, mood changes, increased thirst, loss of appetite; swelling, weight gain, shortness of breath; seizures (convulsions); breathing problems; pounding heartbeats or fluttering in your chest; or severe pain, burning, or irritation around the IV needle.
Dosage
Gadobutrol is given as an infusion into a vein. The recommended dose of Gadobutrol for adult and pediatric patients (including term neonates) is 0.1 mL/kg body weight (0.1 mmol/kg).
Waste Disposal
Expired or waste pharmaceuticals shall carefully take into consideration
applicable DEA, EPA, and FDA regulations. It is not appropriate to
dispose by flushing the pharmaceutical down the toilet or discarding to
trash. If possible return the pharmaceutical to the manufacturer for
proper disposal being careful to properly label and securely package the
material. Alternatively, the waste pharmaceutical shall be labeled,
securely packaged and transported by a state licensed medical waste
contractor to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator.