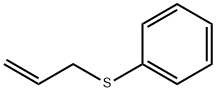
Synthesis Reference(s)
Synthesis, p. 625, 1984
Tetrahedron, 47, p. 8621, 1991
DOI: 10.1016/S0040-4020(01)82405-4Tetrahedron Letters, 20, p. 1481, 1979
Purification Methods
Dissolve the sulfide in Et2O, wash with alkali, H2O, dry over CaCl2, evaporate and fractionally distil it, preferably under vacuum. It should not give a precipitate with an alcoholic solution of Pb(OAc)2. [Hurd & Greengard J Am Chem Soc 52 3356 1930, Tarbell & McCall J Am Chem Soc 74 48 1952, Beilstein 6 IV 1479.]
Preparation and handling
ALLYL PHENYL SULFIDE can be made from Allyl Bromide and sodium thiophenolate in ethanol.
Handling, Storage, and Precautions: must be handled in a fume hood due to stench caused by contamination with Thiophenol. The pure sulfide has a more agreeable odor with a sweet overtone. No special precautions are necessary when handling in the atmosphere for weighing and transferring. In order to avoid autoxidation the sulfide should be stored in a well sealed vessel with exclusion of oxygen and moisture at 4 °C.