Manufacturing Process
To 3-(3-methyl-1-piperazinyl)-4-fluoro-5-methyl-6-nitro-N-cyclopropylaniline is
added diethyl ethoxymethylenemalonate and the mixture is heated at 150°C
for 25 hours. After cooling, the reaction product is purified by silica-gel
column-chromatography (dichloromethane:methanol = 100:1) to give
diethyl[N-cyclopropyl-N-[3-(3-methyl-1-piperazinyl)-4-fluoro-5-methyl-6-nitrophenyl] aminomethylene]malonate. The product is dissolved in acetic
anhydride and thereto conc. sulfuric acid is added dropwise at 50-60°C,
followed by stirring for 30 min. The mixture is poured into ice-water,
neutralized, extracted with dichloromethane and the extract is dried. The
solvent is distilled off under reduced pressure. Purification by silica-gel
column-chromatography (dichloromethane:methanol = 10:1) to give ethyl 7-
(3-methyl-1-piperazinyl)-1-cyclopropyl-6-fluoro-5-methyl-1,4-dihydro-4-
oxoquinoline-3-carboxylate. To these compound is 10% aqueous solution of
sodium hydroxide and ethanol, and the mixture is refluxed for 1 hour. After
cooling, the reaction mixture is diluted with water and washed with
dichloromethane. The aqueous layer is made acidic with acetic acid and then
made weakly alkaline with an aqueous sodium hydrogen carbonate. The
product is extracted with dichloromethane and the extract is dried. The
solvent is distilled off under reduced pressure and to the residue is added
ethanol. The precipitated crystals are filtered and recrystallized from DMFA to
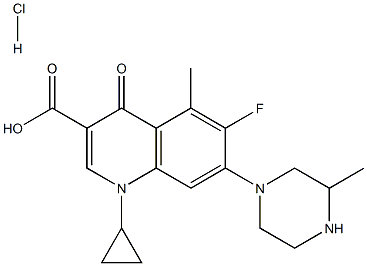
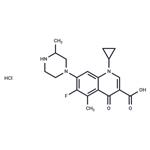
give 7-(3-methyl-1-piperazinyl)-1-cyclopropyl-6-fluoro-5-methyl-1,4-dihydro-
4-oxoquinoline-3-carboxylic acid (12 mg), as white powder, m.p. 206-208°C.
7-(3-methyl-1-piperazinyl)-1-cyclopropyl-6-fluoro-5-methyl-1,4-dihydro-4-
oxoquinoline-3-carboxylicacid may be transformed to hydrochloride.