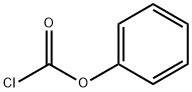
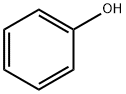
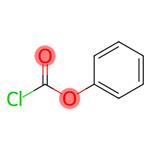
Chemical Properties
clear oily liquid. Corrosive. Insoluble in water, soluble in ethanol, ether, easily soluble in petroleum ether.
Uses
Phenyl chloroformate is used in the synthesis of poly(2-(phenoxycarbonyloxy)ethyl methacrylate) and phenyl-(4-vinylphenyl) carbonate. It acts as a precursor of phenyl mixed anhydrides which are used in peptide coupling reactions. It serves as a dehydrating reagent for the conversion of primary amides to nitriles and an intermediate in the synthesis of pharmaceuticals and carbamates.
Preparation
Phenyl chloroformate is synthesized by the reaction of phenol with phosgene. Phenol was dissolved in chloroform, phosgene was introduced under cooling, and the absorbed phosgene was in an equal molar ratio to phenol, and equimolar N,N-dimethylaniline was added dropwise under stirring at 5-10 °C. Then add cold water to dilute, separate the oil layer, wash with dilute hydrochloric acid and water successively. After drying with anhydrous calcium chloride, chloroform is evaporated, and then distilled under reduced pressure to collect 74-75°C (1.73kPa) fraction, which is phenyl chloroformate. The yield is about 90%.
Application
Phenyl chloroformate is an important organic synthesis intermediate, which is widely used in chemical synthesis and can be used as polymer catalyst, plastic modifier, fiber treatment agent, and intermediate of medicine and pesticide.
General Description
Phenyl chloroformate appears as a colorless liquid with a strong odor. Toxic by ingestion, inhalation and skin absorption. Very irritating to skin and eyes. Used as a reagent for organic synthesis.
Air & Water Reactions
Emits fumes containing HCl in moist air. Decomposes in water to form HCl.
Reactivity Profile
Phenyl chloroformate is incompatible with strong oxidizing agents, alcohols, amines, alkali. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by inhalation.
Moderately toxic by ingestion and skin
contact. A corrosive sktn and eye irritant.
See also ESTERS. When heated to
decomposition it emits toxic fumes of Cl-.