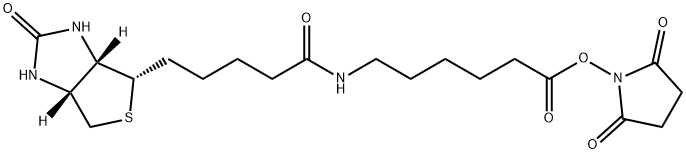
Description
Biotin-LC-NHS Ester has an extended spacer arm which helps to minimize steric hindrance. The membrane permeability of this reagent allows it to be used for intracellular labeling. It can react efficiently with primary amino (-NH2) to form stable, irreversible amide bonds.
Chemical Properties
Succinimidyl 6-(biotinamido)hexanoate is White Solid
Uses
Succinimidyl 6-(biotinamido)hexanoate is a biotinylation reagent incorporating an aminocaproyl “spacer”. This can reduce the steric hindrance in binding avidin to some biotinylated compounds when used to biotinylate both the antibody and the carrier in the Protein Avidin-Biotin Capture System. This system avoids the direct interaction of a captured antibody with solid surfaces, such as plastics, which may reduce antigenicity. It has also been used to enhance the detection of DNA on nitrocellulose. Has also been used as a cell surface labelling reagent
Uses
Has been used to biotinylate both the antibody and the carrier in the Protein Avidin-Biotin solid surfaces, such as plastics, which may reduce antigenicity. It has also been used to enhance the detection of DNA on nitrocellulose.
Spacer Arm:
Uses
A biotinylation reagent incorporating an aminocaproyl pacer? This can reduce the steric hindrance in binding avidin to some biotinylated compounds when used to biotinylate both the antibody and the carrier in the Protein Avidin-Biotin Capture Sy
Biological Activity
nhs-lc-biotin (succinimidyl-6-(biotinamindo)hexanoate), also known as nhs-x-biotin is a derivative of d-biotin, a amine-reactive biotinylation agent, that contains a spacer arm off the valeric acid side chain of d-biotin with an nhs ester group at its end. the nhs ester group at the end of nhs-lc-biotin covalently binds to amine groups in proteins and other molecules forming a stable amide linkage and releasing the nhs group. the 6-aminocaproic acid spacer of nhs-lc-biotin greatly increases the length between a covalently modified molecule and the bicyclic biotin rings leading to a better binding potential for avidin or streptavidin probes. nhs-lc-biotin is insoluble in aqueous environments requiring the dissolution of organic solvents prior to the addition to a buffered reaction.bioconjugate techniques , 2nd ed. by greg t.hermanson (pierce biotechnology, thermo fisher scientific, rockford, il). academic press (an imprint of elsevier): london, amsterdam, burlington, san diego . 2008. isbn 978-0-12-370501-3.
Purification Methods
Dissolve ~400mg of the ester in dry propan-2-ol (~25mL) with gentle heating. Reduce the volume to ~10mL by gentle boiling and allow the solution to cool. Decant the supernatant carefully from the white crystals, dry the crystals in a vacuum over P2O5 at 60o overnight. This material gives one spot on TLC. [Costello et al. Clin Chem 25 1572 1979, Kincaid et al. Methods Enzymol 159 619 1988.]