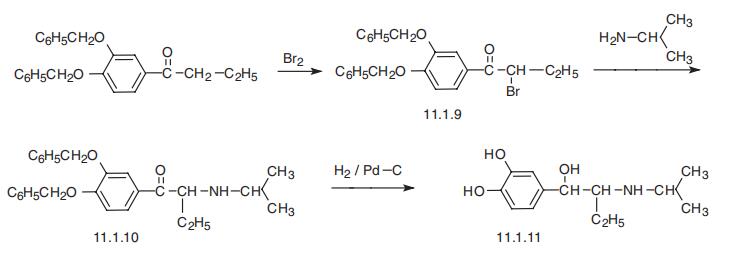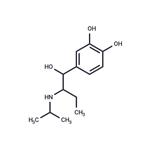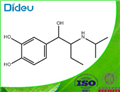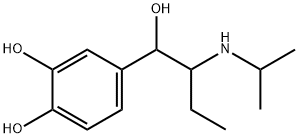
isoetarine
- Product Nameisoetarine
- CAS530-08-5
- CBNumberCB1920266
- MFC13H21NO3
- MW239.313
- EINECS208-472-1
- MDL NumberMFCD00867038
- MOL File530-08-5.mol
Chemical Properties
| Boiling point | 429.3±40.0 °C(Predicted) |
| Density | 1.142±0.06 g/cm3(Predicted) |
| pka | 9.59±0.10(Predicted) |
| CAS DataBase Reference | 530-08-5 |
| FDA UNII | YV0SN3276Q |
| ATC code | R03AC07,R03CC06 |