Description
Triflupromazine is a phenothiazine with diverse biological activities. It binds to muscarinic receptors in isolated rat corpus striatum (IC
50 = 100 μM in a radioligand binding assay). Triflupromazine inhibits serotonin (5-HT) uptake by isolated rat brainstem synaptosomes (IC
50 = 0.8 μM). It inhibits
T. cruzi infection in mouse peritoneal macrophages when used at a concentration of 12.5 μM. Triflupromazine is active against
S. aureus, shigellae, and vibrios (MICs = 2-100 μg/ml)
in vitro and is protective against
S. typhimurium infection in mice when administered at a dose of 30 μg per animal. Formulations containing triflupromazine were previously used as antipsychotics.
Originator
Vesprin,Squibb,US,1957
Uses
analgesic, antiinflammatory, antipyretic, COX-II inhibitor
Manufacturing Process
Approximately 3.8 grams of sodamide is freshly prepared from 2.25 grams of
sodium, 90 grams of liquid ammonia and a catalytic trace of ferric nitrate. The
ammonia is allowed to evaporate. A solution of 19.1 grams of 2-trifluoromethylphenothiazine (prepared by the Bernthsen thionation of 3-
trifluoromethyldiphenylamine) in 160 ml of dry benzene is added to the
reaction flask followed by 18 grams of 3-chloro-1-dimethylaminopropane. The
reaction mixture is heated at reflux for 20 hours. After washing the cooled
mixture with 130 ml of water, the organic layer is extracted with several
portions of dilute hydrochloric acid. The acid extracts are combined and
neutralized with ammonium hydroxide solution. The oily free base is extracted
into benzene and purified by distillation to give 19.6 grams of 10-(3'-
dimethylaminopropyl)-2-trifluoromethylphenothiazine, boiling point 177° to
181°C at 1 mm. The free base (7 grams) is converted to the hydrochloride
salt by reacting an alcoholic solution of the base with hydrogen chloride gas.
Evaporation of the volatiles in vacuo leaves an amorphous solid which is
recrystallized from ethanol/ether to pink crystals, MP 173° to 174°C, the
hydrochloride salt of the free base prepared above.
brand name
Vesprin (Bristol-Myers Squibb).
Therapeutic Function
Tranquilizer
General Description
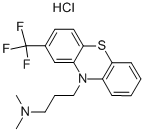
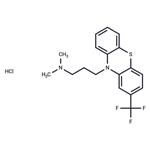
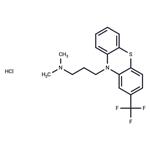
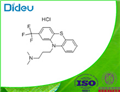
Triflupromazinehydrochloride, 10-[3-(dimethylamino)propyl]-2-(trifluoromethyl)phenothiazine monohydrochloride (Vesprin), has agreater milligram potency as an antipsychotic, higher EPS,but lower sedative and hypotensive effects than chlorpromazine.The 2-CF
3 versus the 2-Cl is associated with thesechanges. Overall, the drug has uses analogous to those ofchlorpromazine.