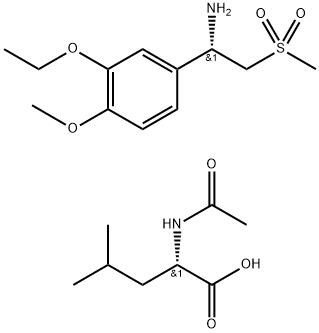
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt
- Product Name(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt
- CAS608141-43-1
- CBNumberCB12707852
- MFC20H34N2O7S
- MW446.56
- EINECS-0
- MDL NumberMFCD28167941
- MOL File608141-43-1.mol
- MSDS FileSDS
Chemical Properties
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| CAS DataBase Reference | 608141-43-1 |
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| TRC E898128 | 500mg | $60 | (S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamineN-Acetyl-L-leucineSalt |
Buy |
| ChemScene CS-M2810 | 10g | $70 | (S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamineN-acetyl-L-leucinesalt 99.99% |
Buy |
| ChemScene CS-M2810 | 100g | $375 | (S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamineN-acetyl-L-leucinesalt 99.99% |
Buy |
| Activate Scientific AS93594 | 25g | $443 | (S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine N-Acetyl-L-leucine Salt 95+% ee |
Buy |
| Chemenu CM186592 | 10g | $130 | (S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethan-1-amineacetyl-L-leucinate 95+% |
Buy |
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt Chemical Properties,Usage,Production
Synthesis
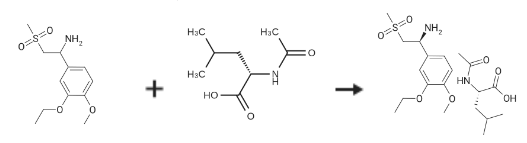
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt is prepared by the reaction of 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine and N-Acetyl-L-leucine. The specific synthesis steps are as follows:A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with 2-(3 -ethoxy-4-methoxyphenyl)- 1 -(methylsulphonyl)-eth-2-ylamine (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered andwashed with methanol (250 mL). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98percent yield) of the crude product (85.8percent ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried invacuo at 30°C. to a constant weight, yielding 49.6 g (90percent recovery) of(S)-2-(3-ethoxy-4-methoxyphenyl)- 1 -(methylsulphonyl)-eth-2-ylamine-N-acety l-L-leucine salt (98.4percent ee). ChiralHPLC (1/99 EtOH/20 mM KH2PO4 pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies,150 mm4.6 mm, 0.5 mL/min., 240 nm): 18.4 mm (S-isomer, 99.2percent), 25.5 mm (R-isomer,0.8percent).
Preparation Products And Raw materials
Preparation Products
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt Supplier
Global(186)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-0571-28186870; +undefined8613073685410 |
sales@ichemie.com | China | 1015 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29792 | 60 | |
| +86-371-66670886 | info@dakenam.com | China | 19902 | 58 | |
| 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 | |
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29871 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 | |
| 21-33585366 | export01@shyrchem.com | CHINA | 1319 | 58 | |
| +8615318812076 | zpy8217@163.com | China | 286 | 58 | |
| +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
View Lastest Price from (S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt manufacturers
608141-43-1, (S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine saltRelated Search
- Apremilast Impurity 9
- Apremilast Impurity 2
- APST-ZA
- (R)-N-(2-(1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide
- (S)-N-(2-(1-(3-ethoxy-4-hydroxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine