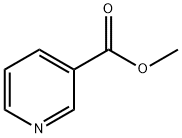
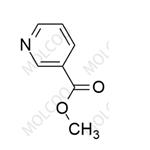
Description
Methyl nicotinate is a skin irritant in man, in particular producing vasodilatation, which is used on purpose in “rubefacient”, to produce a redness or inflammation for a short period of time, often in lip plumper or other lip products. Methyl nicotinate is also an active ingredient of a number of veterinary preparations intended for topical application as a rubefacient for the treatment of a variety of diseases, such as respiratory diseases, vascular disorders (oedema, haematoma), and rheumatoid disorders in cattle and horses (doses not stated; the concentration in the formulated veterinary medicinal product is 2%).
Chemical Properties
Methyl nicotinate is white to brownish crystals or crystalline powder and has a fresh, caramellic, nutty and mild tobacco odor.
Occurrence
Reported found in ground coffee, roasted filberts, guanabana (Annona muicata L.), roasted peanuts, wild
strawberries, vanilla bean, papaya, guava, plum, beer cognac, soursop, malt, wort, Bourbon vanilla and mountain papaya.
Uses
Methyl nicotinate (MN) is the methyl ester of nicotinic acid that is used as an active ingredient, a rubefacient and counter-irritant in over-the-counter topical preparations indicated for the temporary relief of aches and pains in muscles,tendons, and joints. The action of methyl nicotinate as a rubefacient is thought to involve peripheral vasodilation.For veterinary purposes,methyl nicotinate is used to treat respiratory diseases, vascular disorders, rheumatoid arthritis, and muscle and joint pains.Methyl nicotinate can be found in in guava fruit, papaya, strawberry, soursop(Annona muricata), beer, grape brandy, coffee, roasted filbert, roasted peanut and Bourbon vanilla.
Application
Methyl nicotinate can be employed as a precursor in the synthesis:
Di-3-pyridyl ketone ligand, which is used in the preparation of silver(I) complexes for the derivation of coordination polymeric chains.
5-arylnicotinates , ±-sesbanine , and 1,4-dihydropyridine derivatives.
Preparation
Methyl nicotinate was prepared by esterification of nicotinic acid by refluxing with methanol in the presence of concentrated sulphuric acid, esterification product obtained was extracted into organic solvent (chloroform) after neutralization of the reaction mixture with 10% sodium bicarbonate.
Synthesis and antinociceptive activity of methyl nicotinate
DOI:
10.4314/jpb.v12i1.8
Definition
ChEBI: Methyl nicotinate is a member of pyridines and an aromatic carboxylic acid.
General Description
Methyl nicotinate (or nicotinic acid methyl ester) is used as a rubefacient for the relief of pains in muscles, tendons, and joints. It is also used in food as a flavoring agent.
Side effects
There is presently no side effect information available for Methyl nicotinate(MN) itself. a few patients who applied a medicated topical preparation containing MN (and other components) suffered adverse reactions including faintness, nausea, pain and skin rashes.
Safety
No data are available for chronic toxicity of Methyl nicotinate(MN) in humans. No adverse effects were reported for nicotinamide in clinical studies, whereas high doses of NA (3000 mg/day) are associated with considerable toxicity. In one third to one half of the 1119 patients taking 3000 mg NA /day for at least five years (to reduce the incidence of a second myocardial infarction), side effects included gastrointestinal and urinary tract problems, as well as dermatological problems of flushing, itching and rash ("The Coronary Drug Project", 1975, cited in "monograph of methyl nicotinate", Council of Europe, 2008, pp. 248).
Synthesis
Methyl nicotinate is synthesized from Nicotinyl chloride hydrochloride and and Methanol in presence of Pyridine.
Carcinogenicity
No data on the mutagenic or carcinogenic potential of methyl nicotinate were presented. However life-time studies in mice employing doses of 2 to 3 mg nicotinamide/kg bw/day gave no indication of carcinogenic potential.