Description
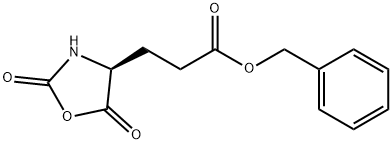
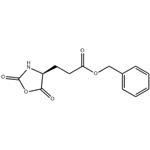
5-Benzyl L-glutamate N-carboxyanhydride is a reactive derivative of L-glutamic acid, where the amino acid has been cyclised as an N-carboxyanhydride (NCA), a class of reagent known as Leuchs’ anhydrides. 5-Benzyl L-glutamate N-carboxyanhydride reacts with nucleophiles such as alcohols and amines to form esters and amides respectively. The opening of the N-carboxyanhydride to an ester or amide releases the amino acid amine which can also react as a nucleophile leading to polymerisation. These poly-amide polymers have been investigated as delivery platforms of Small Interfering RNA (si-RNA).
Uses
(4S)-2,5-Dioxo-4-oxazolidinepropanoic Acid Phenylmethyl Ester was used in the synthesis and physicochemical characterization of reduction-sensitive block copolymer for intracellular delivery of doxorubicin.
Uses
H-GLU(OBZL)-NCA was used in the synthesis and physicochemical characterization of reduction-sensitive block copolymer for intracellular delivery of doxoru bicin.
Synthesis
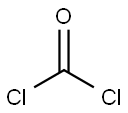
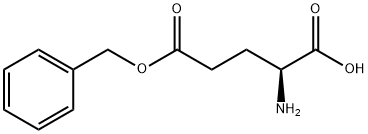
5-Benzyl L-glutamate N-carboxyanhydride (γ-benzyl-L-glutamate-N-carboxyanhydride)was synthesized by a reaction between γ-benzyl-L-glutamate (BLG) and triphosgene. Briefly, 14 g BLG was dissolved in 200 mL anhydrous THF in reaction vessel with condensing reflux unit at stirring state. Then the solution was heated to 50 °C, and 20 g triphosgene was added. The reaction continued until the solution turned from cloudy to clear. The reaction was cooled to room temperature, and N2 was concurrently inlet into the reaction system until the liquid volume did not decrease any more. The solution was precipitated by n-hexane. After being purified by EA/n-hexane and dried in a vacuum oven, 5-Benzyl L-glutamate N-carboxyanhydride was obtained and characterized by 1H nuclear magnetic resonance (1H NMR, Bruker, AV300) using CDCl3 as a solvent[1].
Synthesis
[1] Chen P, et al. Development of Light-Responsive Poly(γ-Benzyl-L-Glutamate) as Photo Switches by a One-Step NCA Method. Frontiers in Chemistry, 2020; 8.