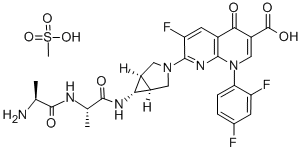
Enzyme inhibitor
This fourth-generation fluoroquinolone-class antibiotic (FW = 558.51 g/mol; CAS 146961-76-4; quickly hydrolyzes to form trovafloxacin), also named 7-[(1R,5S)-6-{[(2S)-1-{[(2S)-2-aminopropanoyl]amino}-1-oxopropan- 2-yl]amino}-3-azabicyclo[3.1.0]hexan-3-yl]-1-(2,4-difluorophenyl)-6- fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid, is a systemic antibacterial that targets Type II DNA topoisomerases (gyrases) required for bacterial replication and transcription. This fluoroquinolone shows an extensive in vitro antibiotic spectrum, with high activity against Grampositive coccus, anaerobic and atypical pneumonia-producing bacteria. (For the prototypical member of this antibiotic class, See Ciprofloxacin) Intravenous alatrofloxacin, followed by oral trovafloxacin (TVX), is safe and well tolerated. Alatrofloxacin also lacks the phototoxicity, cardiovascular toxicity, and hemolytic anemia associated with earlier fluoroquinolones. Given its metabolism to trovafloxacin, it should be noted that the latter significantly increases the formation of nitrotyrosine in mice that are heterozygous for mitochondrial superoxide dismutase-2. Using the NO-selective probe DAF-2, TVX also increases the production of mitochondrial NO in immortalized human hepatocytes. Similarly, mitochondrial Ca2+ is increased by TVX, suggesting calcium ion-dependent activation of mitochondrial NOS activity. The relationship between these observations and trovafloxacin-induced leukopenia remains to be explored.