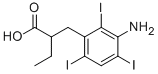
Originator
Telepaque,Winthrop,US,1952
Manufacturing Process
(A) Preparation of α-Ethyl-m-Nitrocinnamic Acid: This acid is prepared from
100 g of m-nitrobenzaldehyde, 210 g of butyric anhydride and 73 g of sodium
butyrate. The crude α-ethyl-m-nitrocinnamicacid is crystallized from ethanol
giving about 105 g, MP 140° to 142°C. From the filtrates there may be
isolated a small amount of a stereoisomer, which when pure melts at 105° to
106°C.
(B) Preparation of m-Amino-α-Ethylhydrocinnamic Acid: A mixture of 50 g of
α-ethyl-m-nitrocinnamic acid, 9.1 g of sodium hydroxide, 600 cc of water and
5 teaspoons of Raney nickel catalyst is shaken at 32°C in an atmosphere ofhydrogen at an initial pressure of 450 psi until the calculated amount of
hydrogen is absorbed. The filtered solution is acidified with hydrochloric acid,
made basic with ammonium hydroxide and again acidified with acetic acid.
Upon concentration of this solution, an oil separates which crystallizes upon
standing, giving about 20 g, MP 60° to 68°C. Complete evaporation of the
filtrate and extraction of the residue of inorganic salts with ether gives about
20 g of additional material, MP 54° to 59°C. Recrystallization of the combined
product from benzene petroleum ether gives about 35 g of m-amino-α-
ethylhydrocinnamic acid, MP 67° to 70°C.
(C) Preparation of β-(3-Amino-2,4,6-Triiodophenyll-α-Ethylpropionic Acid: A
solution of 5.0 g of m-amino-α-ethylhydrocinnamic acid in 100 cc of water
containing 5 cc of concentrated hydrochloric acid is added over a period of ?
hour to a stirred solution of 3.2 cc of iodine monochloride in 25 cc of water
and 25 cc of concentrated hydrochloric acid heated to 60°C. After addition is
complete, the heating is continued for one hour longer at 60° to 70°C. A black
oil separates which gradually solidifies.
The mixture is then cooled and sodium bisulfite added to decolorize.
Recrystallization of the product from methanol gives about 8 g, MP 147° to
150°C. The β-(3-amino-2,4,6-triiodophenyl)-α-ethylpropionic acid may be
purified further by precipitation of the morpholine salt from ether solution and
regeneration of the free amino acid by treatment of a methanol solution of the
morpholine salt with sulfur dioxide. The pure amino acid has the MP 155° to
156.5°C.