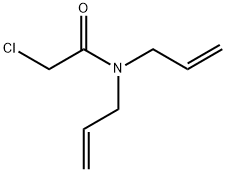
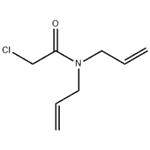
Chemical Properties
An oily, amber liquid. Slightly irritating odor
Chemical Properties
Amber liquid or granules.Slightly soluble in water; soluble in alcohol, hexane, and xylene.
Uses
Selective preemergence herbicide used to control annual grass weeds and some
broad-leaved weeds in maize, millet, soybeans, sorghum, sugarcane, vegetables and ornamentals.
Uses
Pre-emergent herbicide.
Definition
An amide that modi-
fies RNA and protein biosynthesis and inhibits cell
division in primary meristems.
General Description
Amber liquid.
Air & Water Reactions
Slightly water soluble.
Reactivity Profile
Corrosive to steel. .
Hazard
Toxic by ingestion. Dry formulations are irritating to eyes and skin.
Fire Hazard
Flash point data for ALLIDOCHLOR are not available. ALLIDOCHLOR is probably not flammable.
Safety Profile
Poison by skin contact.
Moderately toxic by ingestion. An herbicide.
When heated to decomposition it emits very
toxic fumes of Cland NOx. See also
ALLYL COMPOUNDS.
Potential Exposure
Acetamide, and organochlorine herbicide, primarily used to control weeds growing in onion crops. Used as a preemergence and postemergence control for most annual grasses and broadleaf weeds on corn, sorghum, lima beans, snap beans, soybeans, cabbage, peas for canning, celery, onions and some fruits and ornamentals. There are no products registered with the United States Environmental Protection Agency revoked all tolerances on July 21, 1999. There are 25 manufacturers worldwide with 6 located in the U.S.
Environmental Fate
Plant. Allidochlor is translocated in plants to chloroacetic acid and diallylamine. The
diallylamine is further transformed to carbon dioxide. The acid undergoes further degradation to glycollic acid which breaks down to glyoxalic acid. Glyoxalic acid undergoes
further degradation to give formic acid, glycine and carbon dioxide (Cremlyn, 1991)
Chemical/Physical. Emits very toxic fumes of phosphorus oxides and chlorine when
heated to decomposition (Sax and Lewis, 1987).
Shipping
UN2996 Organochlorine pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2902 Pesticide, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Incompatibilities
Oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); strong acids. Slowly hydrolyzes in water, releasing ammonia and forming acetate salts. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Waste Disposal
Organochlorines may be completely dechlorinated by sodium in isopropyl alcohol. The UN Recommends incineration methods for disposal of organochlorines. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.