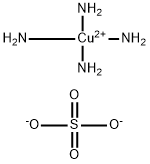
Chemical Properties
dark blue crystal(s); odor of ammonia; decomposes in air; made by dissolving CuSO4 in water containing ammonia, then by precipitating with ethanol; used in textile printing and as a fungicide [MER06]
General Description
A dark blue crystalline solid with a faint odor of ammonia. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used as a pesticide, to print fabrics, and to make other copper compounds.
Air & Water Reactions
Soluble in water.
Reactivity Profile
TETRAAMMINECOPPER (II) SULFATE HYDRATE gives off ammonia when heated to 120°C.
Health Hazard
INHALATION: Inhalation of dust may produce severe irritation of upper respiratory tract. Congestion of the nasal mucosa. EYES: May cause conjunctivitis and edema of eyelids. SKIN: May cause irritation. INGESTION: May induce severe gastroenteric distress: vomiting, pain, local corrosion, and hemorrhages.
Fire Hazard
Special Hazards of Combustion Products: Gives off ammonia when heated to 120°C.