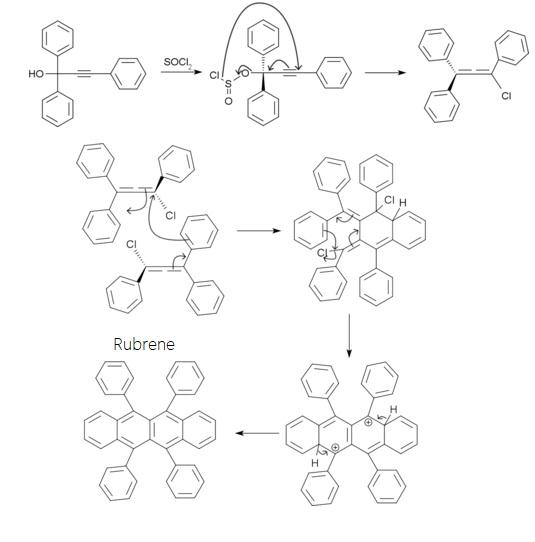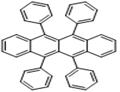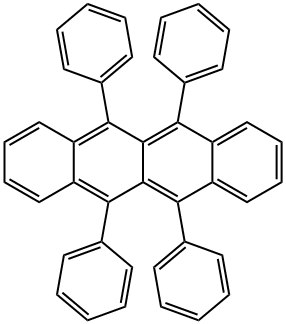
Rubrene
- Product NameRubrene
- CAS517-51-1
- CBNumberCB0384519
- MFC42H28
- MW532.67
- EINECS208-242-0
- MDL NumberMFCD00003703
- MOL File517-51-1.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 330-335 °C (lit.) |
| Boiling point | >315°C |
| Density | 1.1750 (estimate) |
| refractive index | 1.7160 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | THF: soluble chloroform: soluble toluene: soluble |
| form | powder |
| color | Red |
| optical activity | [α]/D |
| Water Solubility | Soluble in hot toluene. Insoluble in water. |
| Sensitive | Air Sensitive |
| λmax | 299 nm 460 nm (2nd) |
| BRN | 1917339 |
| InChIKey | YYMBJDOZVAITBP-UHFFFAOYSA-N |
| CAS DataBase Reference | 517-51-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 5,6,11,12-Tetraphenylnaphthacene(517-51-1) |
| EPA Substance Registry System | Naphthacene, 5,6,11,12-tetraphenyl- (517-51-1) |
| Absorption | λmax?299 nm (in THF) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H335-H315-H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29029090 | |||||||||
| NFPA 704: |
|