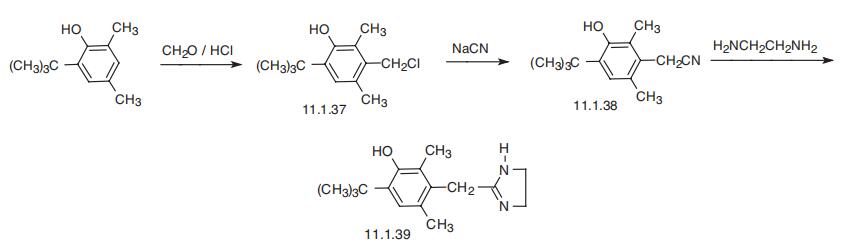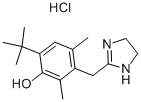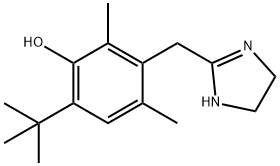
Oxymetazoline
- Product NameOxymetazoline
- CAS1491-59-4
- CBNumberCB0371500
- MFC16H24N2O
- MW260.37
- EINECS216-079-1
- MDL NumberMFCD00242798
- MOL File1491-59-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 182 °C |
| Boiling point | 403.63°C (rough estimate) |
| Density | 0.9822 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| pka | 11.93±0.28(Predicted) |
| color | Crystals from C6H6 |
| CAS DataBase Reference | 1491-59-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8VLN5B44ZY |
| ATC code | R01AA05,R01AB07,S01GA04 |
| NIST Chemistry Reference | Oxymetazoline(1491-59-4) |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H412-H318-H300-H330 |
| Precautionary statements | P273-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P280-P305+P351+P338-P310-P264-P270-P301+P310-P321-P330-P405-P501 |
| RIDADR | 3249 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| Hazardous Substances Data | 1491-59-4(Hazardous Substances Data) |
| Toxicity | LD50 orl-rat: 800 mg/kg TXAPA9 18,185,71 |