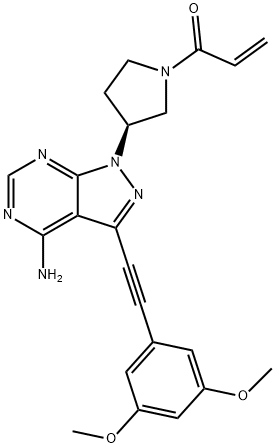
Binding Mode
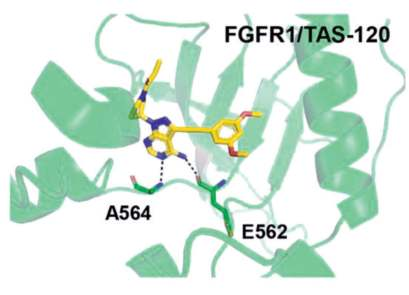
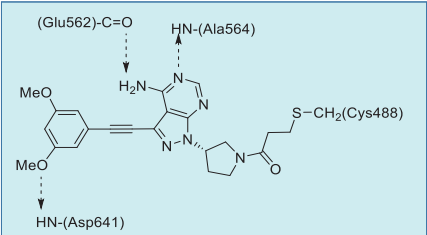
In the co-crystal structure with FGFR1,2,3 the
pyrazolopyrimidine core forms two hydrogen bonds
to the amide NH of Ala564 and amide oxygen of
Glu562 of the hinge region. The dimethoxyphenyl
ring, similarly to other FGFR1-selective inhibitors,
occupies a conserved hydrophobic pocket, forming
a single hydrogen bond to Asp641. Most importantly,
the inhibitor is irreversibly attached to the protein via
a covalent bond between the acrylamide group and
the reactive P-loop Cys488 (Figs. 1, 2).
Uses
Futibatinib is an orally bioavailable inhibitor of the fibroblast growth factor receptor (FGFR) with potential antineoplastic activity. Futibatinib selectively and irreversibly binds to and inhibits FGFR, which may result in the inhibition of both the FGFR-mediated signal transduction pathway and tumor cell proliferation, and increased cell death in FGFR-overexpressing tumor cells. FGFR is a receptor tyrosine kinase essential to tumor cell proliferation, differentiation and survival and its expression is upregulated in many tumor cell types.
General Description
Class: receptor tyrosine kinase;
Treatment: Cholangiocarcinoma;
Other name: TAS 120;
Elimination half-life = 2.9 h;
Protein binding = 95%
Synthesis
The synthesis of Futibatinib is as follows:
2 methanesulfonate (25.0 g), water (69 mL), and acetonitrile (158 mL) were placed in a reaction vessel, and a 5N aqueous sodium hydroxide solution (35 mL) was added thereto. A solution prepared by diluting 3-chloropropionyl chloride (6.27 g) with acetonitrile (50 mL) was added over a period of 10 minutes. After completion of the dropwise addition, the mixture was stirred at 30° C. for 30 minutes. The reaction solution was partially taken out and measured by HPLC (condition 3). The peak area of compound B was confirmed to be less than 0.1% of the total peak area. At this stage, the diamide compound and the 3CP diamide compound were not detected in HPLC. Thereafter, a 5N aqueous sodium hydroxide solution (25 mL) was further added, and the mixture was stirred at 30° C. for 4 hours. The reaction solution was partially taken out and measured by HPLC (condition 3). The peak area of the A-1-3CP compound was confirmed to be less than 0.1% of the total peak area. After completion of the reaction, water (550 mL) was added over a period of 2 hours. After completion of the dropwise addition, the internal temperature was adjusted to 25° C., and the mixture was stirred for 1.5 hours. The insoluble matter was collected by filtration and washed with water (125 mL), followed by drying the washed matter at 60° C. under reduced pressure, thereby obtaining the Futibatinib (16.02 g, yield 85.3%).
in vitro
Futibatinib (TAS-120) covalently binds to a highly conserved P-loop cysteine residue in the ATP pocket of FGFR.
in vivo
Futibatinib (TAS-120) (3, 30, 100 mg/kg/day, p.o.) exerts an anti-tumor
effect in mice. Futibatinib (TAS-120) shows anti-tumor effect by
administering at moderate intervals, such as intermittent administration
of every other day dosing and 2 times/week, and reducing the sustained
elevation and weight suppression blood phosphorus level, and take a
antitumor effective as daily administration.
target
Primary target: Pan-EGFR