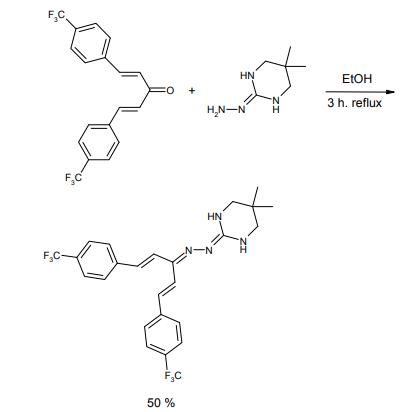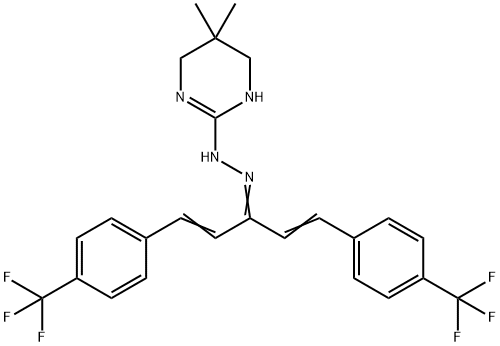
Hydramethylnon
- Product NameHydramethylnon
- CAS67485-29-4
- CBNumberCB0296517
- MFC25H24F6N4
- MW494.48
- EINECS405-090-9
- MDL NumberMFCD00128045
- MOL File67485-29-4.mol
Chemical Properties
| Melting point | 185-190°C |
| Boiling point | 510.5±60.0 °C(Predicted) |
| Density | 1.2816 (estimate) |
| vapor pressure | 2.7 x l0-6 Pa (25 °C) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), DMSO (Sparingly), Methanol (Slightly) |
| Water Solubility | 0.005-0.007 mg l-1(25 °C) |
| pka | 14.38±0.40(Predicted) |
| BRN | 6015162 |
| Stability | Light Sensitive |
| CAS DataBase Reference | 67485-29-4(CAS DataBase Reference) |
| FDA UNII | J265GZ7MFJ |
| Proposition 65 List | Hydramethylnon |
| NIST Chemistry Reference | 2(1H)-pyrimidinone, tetrahydro-5,5-dimethyl-, (3-(4-(trifluoromethyl)phenyl)-1-(2-(4-(trifluoromethyl)phenyl)ethenyl)-2-propenylidene)hydrazone(67485-29-4) |
| Pesticides Freedom of Information Act (FOIA) | Amdro |
| EPA Substance Registry System | Hydramethylnon (67485-29-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
Safety
| Symbol(GHS) |
  
|
| Signal word | Danger |
| Hazard statements | H302-H319-H372-H410 |
| Precautionary statements | P273-P301+P312+P330-P305+P351+P338-P314 |
| Hazard Codes | T;N,N,T |
| Risk Statements | 22-36-48/25-50/53 |
| Safety Statements | 1/2-22-26-36/37-45-60-61 |
| RIDADR | UN3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | UW7583000 |
| Hazardous Substances Data | 67485-29-4(Hazardous Substances Data) |