Description
Decylubiquinone (55486-00-5) inhibits the Ca2+?dependent mitochondrial permeability transition pore (MPTP) without inhibiting respiration.1?Maximum effect on Ca2+?retention capacity of rat liver mitochondria at 100 μM. Inhibits etoposide-induced apoptosis in in L929 cells at 5 μM via inhibition of cytochrome C release.2?Delays rotenone-induced cardiac dysfunction in a zebrafish model of development and cardiovascular function.3?Substrate for various quinone oxidoreductases.4,5
Uses
Decylubiquinone is inhibits the Ca2+-dependent mitochondrial permeability transition pore (MPTP) and also inhibits etoposide-induced apoptosis.
Definition
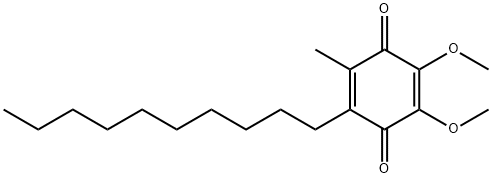
ChEBI: 6-decylubiquinone is a member of the class of 1,4-benzoquinones that is 2,3-dimethoxybenzoquinone which has been substituted at positions 5 and 6 by decyl and methyl groups. It has a role as a cofactor. It is functionally related to a 6-decylubiquinol.
General Description
Ubiquinone analog.
References
1) Fontaine?et al. (1998),?A Ubiquinone-binding Site Regulates the Mitochondrial Permeability Transition Pore; J. Biol. Chem.,?273?25734
2) Karpinich?et al. (2002),?The course of Etoposide-Induced Apoptosis from Damage to DNA and p53 Activation to Mitochondrial Release of Cytochrome c; J. Biol. Chem.,?277?16547
3) Pinho?et al. (2013)?How mitochondrial dysfunction affects zebrafish development and cardiovascular function: an in vivo model for testing mitochondria-targeted drugs; Br. J. Pharmacol.,?169?1072
4) Kunow?et al.?(1994)?F420H2: quinone oxidoreductase from Archaeoglobus fulgidus. Characterization of a membrane-bound multisubunit complex containing FAD and iron-sulfur clusters; Eur. J. Biochem.,?223?503
5) Zickermann?et al?(1998)?Analysis of the pathogenic human mitochondria mutation ND1/3460 and mutations of strictly conserved residues in it’s vicinity, using the bacterium Paracoccis denitrificans; Biochemistry,?37?11792