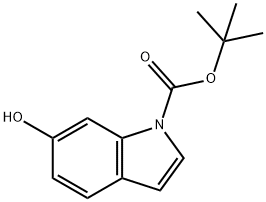
Synthesis
Tert-butyl 6-fluoro-5-hydroxy-1H-indazole-1-carboxylate was prepared as
described for intermediate tert-butyl 5-hydroxy-1H-indazole-1-carboxylate/ tert-butyl 5-hydroxy-2H-indazole-2-
carboxylate replacing tert-butyl 5-((tert-butyldimethylsilyl)oxy)-1H-indazole-1-carboxylate / tert-butyl 5-((tertbutyldimethylsilyl)
oxy)-2H-indazole-2-carboxylate with tert-butyl 5-((tert-butyldimethylsilyl)oxy)-6-fluoro-1H-indazole-1-carboxylate(yield: 94%). 1H
NMR (400 MHz, Chloroform-d) δ 8.06 (d, J = 0.9 Hz, 1H), 7.95 (d, J = 10.7 Hz, 1H), 7.28 (d, J
= 8.2 Hz, 2H), 5.48 (s, 1H), 1.71 (s, 9H).