Uses
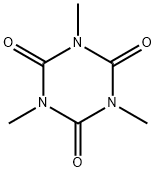
The crystal structure of Trimethyl-1,3,5-triazinane-2,4,6-trione has been reported previously to have low accurancy and without H-atom position, this compound in its molecular complex with 1,3,5-trinitrobenzene has been used in the construction of suprmolecular networks stabilized by hydrogen bonds.
Synthesis Reference(s)
Journal of the American Chemical Society, 78, p. 4358, 1956
DOI: 10.1021/ja01598a043
General Description
Monoclinic prisms (from water or alcohol).
Reactivity Profile
Isocyanates and thioisocyanates, such as trimethyl isocyanurate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Polyurethanes are formed by the condensation reaction of diisocyanates with, for example, ethyl glycol.
Fire Hazard
Flash point data for trimethyl isocyanurate are not available. trimethyl isocyanurate is probably combustible.