Description
Petroleum jelly is a semi-solid mixture of hydrocarbons (with carbon numbers mainly higher than 25),originally promoted as a topical ointment for its healing properties.
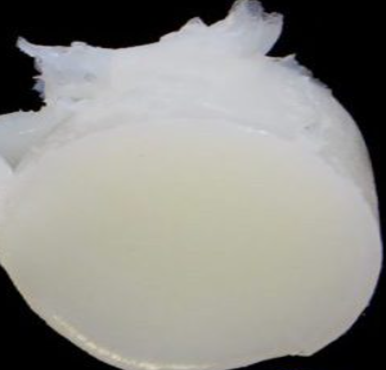
Chemical Properties
Petroleum jelly is a mixture of hydrocarbons, with a melting point that depends on the exact proportions. The melting point is typically between 40 and 70 °C (105 and 160 °F).It is flammable only when heated to liquid; then the fumes will light, not the liquid itself, so a wick material is needed to ignite petroleum jelly.
Physical properties
Petroleum jelly is colorless (or of a pale yellow color when not highly distilled), translucent, and devoid of taste and smell when pure. It does not oxidize on exposure to the air and is not readily acted on by chemical reagents. It is insoluble in water. It is soluble in dichloromethane, chloroform, benzene, diethyl ether, carbon disulfide and turpentine.
Preparation
Petroleum Jelly is obtained by dewaxing heavy lubricating-oil stocks. It has a melting-point range from 38° to 54° C (100° to 130° F). Chemically, petrolatum is a mixture of hydrocarbons, chiefly of the paraffin series.
Application
Relieve dry skin, including your lips and eyelids.
Help injured skin heal.
Prevent chafing.
Treat diaper rash.
Rehydrate nails.
Toxicity
Safe for food (FDA, §172.880,2000).
ADI has not been stipulated (FAO/WHO, 2001).
Health Hazard
Petroleum jelly contains mineral oil aromatic hydrocarbons (MOAH). Many MOAH, mainly polycyclic aromatic hydrocarbons (PAH), are considered carcinogenic.