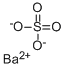
Barium sulfate
- Product NameBarium sulfate
- CAS13462-86-7
- CBNumberCB00131410
- MFBaO4S
- MW233.3896
- EINECS236-664-5
- MOL File13462-86-7.mol
- MSDS FileSDS
Chemical Properties
| Density | 4.300 g/cm3 |
| EWG's Food Scores | 1 |
| FDA UNII | 25BB7EKE2E |
| EPA Substance Registry System | Barite (13462-86-7) |
Barium sulfate Chemical Properties,Usage,Production
Chemical Properties
Barium sulfate is a soft crystalline solid. Its density is 4.50 g/cm3 and its refractive index is 1.64. It melts around 1580 °C but decomposes above 1600 °C. Its hardness is 4.3 to 4.6 Mohs. It is virtually insoluble in water (285 mg/l at 30 °C) and insoluble in alcohol. Its Ksp is 1.1 × 10–10. It is soluble in concentrated sulfuric acid.Physical properties
Barium sulfate is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it.

Uses
Barium sulfate is used to help diagnose or find problems in the esophagus, stomach, and bowels. It is a radiographic contrast agent. Contrast agents are used to create a clear picture of the different parts of the body.Preparation
Barium sulfate, BaSO4, is made by reacting barium hydroxide and other barium sources with sulfuric acid and has a long history as a translucent white pigment.Toxicity
Although soluble salts of barium are moderately toxic to humans, barium sulfate is nontoxic due to its insolubility. The most common means of inadvertent barium poisoning arises from the consumption of soluble barium salts mislabeled as BaSO4.
Structure and conformation
The crystal structure of Barium sulfate is known to be rhombic, with a space group pnma. The lattice parameters are: a = 8.896 Å, b = 5.462°, c = 7.171 Å, V = 348.4 Å3.Preparation Products And Raw materials
Barium sulfate Supplier
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-86-4001020630 +8619831957301 |
admin@hbdangtong.com | China | 999 | 58 | |
| +8615350851019 | admin@86-ss.com | China | 1001 | 58 | |
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21630 | 55 | |
| 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 | |
| +8619521488211 | info@longyupharma.com | China | 2555 | 58 | |
| +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29808 | 58 | |
| +8613367258412 | ada@ipurechemical.com | China | 10244 | 58 | |
| +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7795 | 58 | |
| +86-86-13583358881 +8618560316533 |
Ethan@dornechem.com | China | 3084 | 58 | |
| China | 13194 | 58 |
Related articles
Barite (baryte, barytes; BaSO4) is the most abundant barium mineral in the Earth's crust. It has orthorhombic symmetry, forms good crystals and has good cleavage (perfect along 001). It is relatively insoluble in water.
Jul 14,2022
13462-86-7, Barium sulfateRelated Search
1of4
The What'sApp is temporarily not supported in mainland China

