Description
Strontium carbonate (SrCO3)(1633-05-2) belongs to the carbonate salt of strontium, which is found in nature as the mineral strontianite. It can be applied in a variety of industries. At present, strontium carbonates are commonly being applied as an inexpensive colorant in pyrotechnics since strontium and its salts produce a crimson read flame. Strontium carbonate, in general, is preferred in fireworks, compared with other strontium salts due to its inexpensive cost, nonhygroscopic property, and ability to neutralize acid. It can also be used as road flares and for preparing iridescent glass, luminous paints, strontium oxide or strontium salts and in refining sugar and certain drugs. It is also recommended as a substitute for barium to produce matte glazes. Besides, its applications involves in ceramics industry, where it serves as an ingredient in glazes, and in electric products, where it is used for the production of strontium ferrites to produce permanent magnets for loudspeakers and door magnets. Strontium carbonate is also used for manufacturing some superconductors such as BSCCO and also for electroluminescent materials.
Production Methods
Strontium carbonate occurs in nature as strontianite and can be mined from its deposit. It is, however, usually made from the mineral celestite. Celestite is fused with sodium carbonate at elevated temperatures or boiled with a solution of ammonium carbonate:
SrSO4 + Na2CO3 → SrCO3 + Na2SO4
SrSO4 + (NH4)2CO3 → SrCO3 + 2NH3 + CO2 + H2O
Strontium carbonate is insoluble in water. It precipitates from the product mixture in the second reaction. If fused with sodium carbonate, the product mixture is leached with water. Insoluble carbonate separates from the watersoluble sodium sulfate.
References
https://en.wikipedia.org/wiki/Strontium_carbonate
http://www.nanopartikel.info/en/nanoinfo/materials/strontium-carbonate
Description
Strontium carbonate has the formula of SrCO
3 and
the molecular weight of 147.6326 g/mol. Strontium carbonate occurs in nature as the mineral “strontianite”. The name strontianite
comes from a famous location for the mineral,
Strontian, Scotland. Strontianite is strontium carbonate
as found naturally. It occurs as white or slightly gray orthorhombic
crystals with a refractive index of 1.518. The
unit-cell parameters are: a = 5.107 ? , b = 8.414 ? ,
c = 6.029 ? , Z = 4; V = 259.07 ? 3, Den(Calc) = 3.78. The
crystal system is orthorhombic with space group Pmcn
and point group 2/m, 2/m, 2/m. Strontium carbonate
has only one stable form (aragonite-type structure) and
temperature of precipitation has no effect on crystal
form, unlike that of calcium or magnesium carbonates.
Chemical Properties
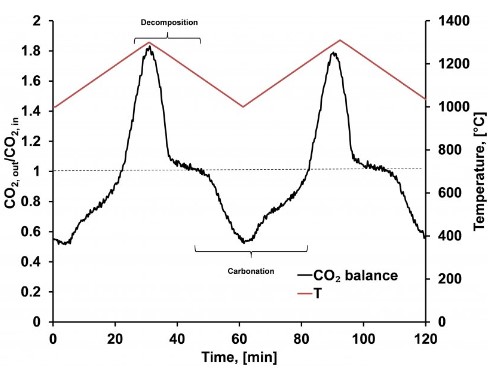
Heating strontium carbonate up from 1000°C to 1300°C causes the compound to undergo a decomposition reaction which results in the capture of thermal energy, shown here as the peak in the black curve occurring between Time = 20 and Time = 40 minutes. Cooling the system back down to 1000°C leads to reconstitution of the starting material in a carbonation reaction thereby releasing the stored thermal energy, a process shown as the valley in the black curve between Time = 50 and Time = 80 minutes.
Chemical Properties
Strontium carbonate is a milky white free flowing powder. It is little more insoluble (Ksol=10-8.8) than calcium carbonate (Ksol=10-8.07), so it should not be surprising that under appropriate conditions Sr2+ can be precipitated by biogenic carbonate.
Physical properties
White orthorhombic crystals; refractive index 1.518; hygroscopic; hardness 3.5 Mohs; density 3.5 g/cm
3; insoluble in water; soluble in dilute acids with liberation of carbon dioxide.
Occurrence
Strontium carbonate occurs in nature as mineral strontianite. The compound is used in pyrotechnics and ceramic ferrites. It also is used in making iridescent glass for color television tubes. Other uses are in refining sugar and preparing other strontium salts.
Uses
Used for electronic applications. It is used for manufacturing CTV to absorb electrons resulting from the cathode
Uses
The compound, SrCO
3, is used in pyrotechnics and
ceramic ferrites. It is also used in making iridescent glass
for color television tubes. Other uses are in refining
sugar and preparing other strontium salts. The most
common use is as an inexpensive fireworks colorant.
Strontium and its salts emit a brilliant red color in flame.
Its ability to neutralize acid is also very
helpful in pyrotechnics. Another similar application is
in road flares. Strontium carbonate is used for electronic
applications. It is used for manufacturing glass colortelevision
tubes to absorb X-rays resulting from the
bombardment of the cathode rays on the glass enclosure
of the cathode-ray gun. SrCO
3 is used in the preparation
of iridescent glass, strontium oxide or strontium salts and in refining sugar.It is widely used in the ceramics industry as an ingredient
in glazes. It acts as a flux and also modifies the
color of certain metallic oxides. It is also used in the
manufacturing of strontium ferrites for permanent
magnets that are used in loudspeakers and
door-magnets. Strontium carbonate can be used to
produce many different strontium compounds by
simply dissolving it in the corresponding acid.
Strontium bicarbonate has not been isolated.
Uses
Used in the preparation of?iridescent glass, luminous paints, strontium oxide or strontium salts and in refining sugar and certain drugs
Uses
Strontium carbonate (SrCO3) is used to make radiation-resistant glass and TV picture tubes, as well as pyrotechnics.
Definition
strontium carbonate: A whitesolid, SrCO
3; orthorhombic; r.d. 3.7;decomposes at 1340°C. It occurs naturallyas the mineral strontianite andis prepared industrially by boiling celestine(strontium sulphate) with ammoniumcarbonate. It can also beprepared by passing carbon dioxideover strontium oxide or hydroxide orby passing the gas through a solutionof strontium salt. It is a phosphor,used to coat the glass of cathode-rayscreens, and is also used in the refiningof sugar, as a slagging agent incertain metal furnaces, and to providea red flame in fireworks.
Preparation
Strontium carbonate occurs in nature as strontianite
and can be mined from its deposit. It is, however,
usually made commercially from the mineral “celestite”.
Celestite is fused with sodium carbonate at elevated
temperatures or boiled with a solution of ammonium
carbonate.Strontium carbonate is insoluble in water. It precipitates
from the product mixture in the second reaction.
If fused with sodium carbonate, the product mixture is
leached with water. Insoluble carbonate separates from
the water-soluble sodium sulfate.
Production Methods
Strontium carbonate, formed (1) by reaction of strontium salt solution and sodium carbonate or bicarbonate solution, (2) by reaction of strontium hydroxide solution and CO2. Strontium carbonate decomposes at 1,200 °C (2,192 °F) to form strontium oxide and CO2, and is dissolved by excess CO2, forming strontium bicarbonate, Sr(HCO3)2, solution.
Definition
strontianite: A mineral form ofstrontium carbonate, SrCO
3.
General Description
Strontium carbonate is insoluble in water. It is used predominantly in producing other strontium salts.
General Description
Strontianite is a strontium carbonate mineral (SrCO
3). It is the original and principal source of strontium. It often occurs in white masses of radiating fibres, although pale green, yellow, or gray colours are also known. Strontianite forms soft, brittle crystals that are commonly associated with barite, celestine, and calcite in low-temperature veins. Strontianite is used in pyrotechnics to impart a red colour and in sugar refining as a clarifying agent.