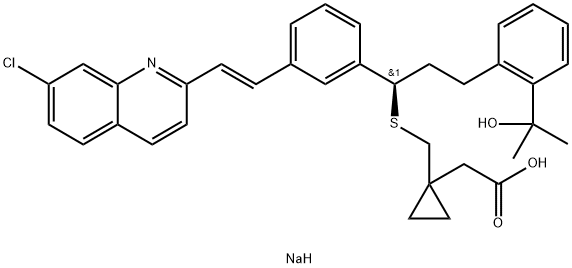
Description
Montelukast was launched as Singulair in Mexico and Finland for the
management of mild to moderate asthma inadequately controlled by inhaled
corticosteroids and short-acting beta2-agonists. Montelukast can be obtained by
an seven-step synthesis from 3-[2(E)-(7-chloroquinolin-2-yl)vinyl] benzaldehyde.
Montelukast is a potent, selective and orally active antagonist of the CysLT1
(formerly called LTD4) receptor, thus blocking the effects of the cysteinyl
leukotrienes LTC4, LTD4 and LTE4 on microvascular permeability and the
activation of eosinophils. Montelukast represents the third molecule of this class
which has been approved in asthma after pranlukast (1995) and zafirlukast
(1996). Montelukast has been studied extensively in placebo-controlled clinical
trials, in mildly or severe asthmatic patients challenged with LTD4 or exercise. A
variety of acute bronchoconstricting challenges were inhibited or attenuated with
all doses used. Montelukast demonstrated clinically significant improvements in
the parameters of asthma control associated with an appreciable improvement
in quality of life., reducing days with asthma exacerbations and allowing
significant tapering of corticosteroids. Montelukast is well-tolerated and only
needs to be administered once a day.
Chemical Properties
White or almost white, hygroscopic powder.
Originator
Merck & Co (US)
Uses
A selective leukotriene D4-receptor antagonist. It is used as an antiasthmatic and It can be also applied to alleviate the symptoms caused by allergic rhinitis.
Uses
Montelukast sodium is a potent and highly selective CysLT1 receptor antagonist, without demonstrated CysLT2 activity. It is used to prevent wheezing, difficulty breathing, chest tightness, and coughing caused by asthma in adults and children 12 months of age and older. Montelukast is also used to prevent bronchospasm (breathing difficulties) during exercise in adults and children 6 years of age and older.
Application
Montelukast sodium hydrate has been used as a positive control drug to study the protective effects of Gumiganghwal-tang aqueous extract (GGTA) against airway inflammation and pulmonary fibrosis.
Indications
Montelukast sodium is an orally active selective leukotriene receptor antagonist that can specifically inhibit the cysteinyl leukotriene receptor. It was successfully developed by the Merck Company (German) and had entered into market in Canada, Finland, and Mexican in 1997. It is suitable for the prevention and long-term treatment of adults and children asthma, including the prevention of daytime and nighttime asthma symptoms, the treatment of asthma patients who are aspirin-sensitive and prevention of exercise-induced bronchial contraction, it can also be used to relieve the seasonal allergic rhinitis symptoms of 15 year-old or over 15 year-old patients whose symptoms are invalid and intolerant to other treatment.
Definition
ChEBI: Montelukast sodium is an organic sodium salt. It contains a montelukast(1-). It is a drug used to treat symptoms of asthma, such as trouble breathing, tight chest, wheezing, coughing, and runny nose. Montelukast sodium blocks the action of a substance that causes airways in the lungs to narrow and causes other symptoms of asthma. It is a type of leukotriene receptor antagonist and a type of antiasthmatic agent. Also called Singulair.
brand name
Singulair (Merck).
Therapeutic Function
Anti-asthmatic
Synthesis Reference(s)
Montelukast sodium is a leukotriene antagonist and inhibits the synthesis of leukotriene biosynthesis.
Process for preparation of montelukast sodium
WO2014001860A1
Biochem/physiol Actions
Montelukast sodium hydrate is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies. It is a subtype specific CysLT1 receptor antagonist.
Pharmacokinetics
Montelukast is a selective leukotriene receptor antagonist and has been approved for the oral administration treatment of asthma and allergic rhinitis. It is also a potent oral preparation that can significantly improve the inflammatory indicators. Biological determination of biochemistry and pharmacology has showed that montelukast sodium has a high affinity and selectivity to the CysLT1 receptors (compared with other kinds of pharmacologically important airway receptors such as prostanoid, cholinergic and β-adrenergic receptors). Montelukast can effectively suppress the physiological effects caused by the binding between LTC4, LTD4 and LTE4 receptor and CysLT1 receptor without any receptor agonistic activity. There is the secondary type of cysteinyl leukotriene receptor (CysLT2) presented in the lungs cysteinyl leukotriene receptor but may be limited to the blood vessels. So far, researchers haven’t cloned two receptors so the situation of CysLT receptor is illustrated through binding assay and pharmacological analysis. It has been now thought that montelukast does not antagonize CysLT2 receptors.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Side effects
Common side effects of montelukast include upper respiratory infection, fever, headache, sore throat, cough, stomach pain, diarrhea, earache or ear infection, flu, runny nose, and sinus infection.
www.mayoclinic.org
Mode of action
Montelukast selectively and competitively blocks the cysteinyl leukotriene 1 (CysLT1) receptor, preventing binding of the inflammatory mediator leukotriene D4 (LTD4).
References
1) Lynch?et al.?(1999),?Characterization of the human cysteinyl leukotriene CysLT1 receptor; Nature,?399?789 DOI:
10.1038/216582) Jones?et al. (1995),?Pharmacology of montelukast sodium (Singulair), a potent and selective leukotriene D4 receptor antagonist; Can. J. Physiol. Pharmacol.,?73?191 DOI:
10.1139/Y95-0283) Reiss?et al.?(1998),?Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group; Arch. Intern. Med.,?158?1213 DOI:
10.1001/ARCHINTE.158.11.12134) Zhao?et al.?(2011),?Montelukast, a cysteinyl leukotriene receptor-1 antagonist, attenuates chronic brain injury after focal cerebral ischaemia in mice and rats; J. Pharm. Pharmacol.,?63?550 DOI:
10.1111/j.2042-7158.2010.01238.x5) Lenz?et al.?(2014),?Cysteinyl leukotriene receptor (CysLT) antagonists decrease pentylenetetrazol-induced seizures and blood-brain barrier dysfunction; Neuroscience,?277?859 DOI:
10.1016/j.neuroscience.2014.07.0586) Huber?et al.?(2011)?Inhibition of leukotriene receptors boosts neural progenitor proliferation; Cell Physiol. Biochem.,?28?793 DOI:
10.1159/000335793