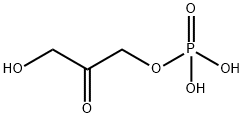
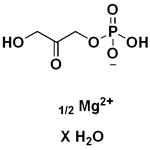
Description
Dihydroxyacetone phosphate (DHAP) is a central player in the metabolism of glucose: It is one of the breakdown products of fructose 1,6-bisphosphate. It also occurs in the Calvin reductive pentose phosphate cycle as one of the products of the NADPH reduction of 1,3-bisphosphoglycerate. DHAP has been used as a three-carbon synthon in the aldolase-mediated synthesis of carbohydrate derivatives.
Biological Functions
Dihydroxyacetone phosphate is a member of the class of glycerone phosphates that consists of glycerone bearing a single phospho substituent. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, and a mouse metabolite. It is a primary alpha-hydroxy ketone and a member of glycerone phosphates. It is functionally related to a dihydroxyacetone.
Biochem/physiol Actions
Dihydroxyacetone phosphate is an intermediate in glycolysis. The first reaction in the dihydroxyacetone phosphate pathway for triacylglycerol synthesis is fatty acylation at position 1 catalyzed by dihydroxyacetone phosphate acyltransferase. Although this enzyme is found in mitochondria, peroxisomes appear to be a more important location. The next step is the reduction of the keto group to form 1-acylglycerol 3-phosphate, linking into the glycerol 3-phosphate pathway of triacylglycerol synthesis. This reduction occurs on the cytosolic side of the peroxisomal membrane and uses NADPH. Further acylation requires transfer of the 1-acylglycerol 3-phosphate to the endoplasmic reticulum.