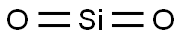Description
Fused silica is a noncrystalline (glass) form of silicon dioxide (quartz, sand). Typical of glasses, it lacks long range order in its atomic structure. It's highly cross linked three dimensional structure gives rise to it's high use temperature and low thermal expansion coefficient.

Characteristics
High chemical purity and resistance
Exceptionally good thermal shock resistance
Can be lapped and polished to fine finishes
Low dielectric constant
Low dielectric loss
Good UV transparency
Uses
Fused silica (SiO2) has very low density, thermal expansion coefficient, electric conductivity, relatively high mechanical property, heat resistance, thermal-shock resistance, corrosion resistance, and dielectric constant, so it is extensively used in optical and optoelectronics devices, microwave dielectric materials, and refractory materials.
Preparation
Fused silica can made by melting some solid form of silica and cooling the melt sufficiently fast to avoid crystallization. A quite high temperature around 1650 to 1700 °C is needed – far above the glass transition temperature of many common optical glasses. The required heat can be provided by an electrically heated furnace or by a flame (Verneuille process) obtained with some combustion gas mixed with pure oxygen.
Forms and nomenclature
Fused silica is sometimes called fused quartz or quartz glass. However, it should be kept in mind that it is an amorphous material, while quartz is crystalline.