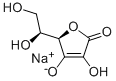
Description
Sodium ascorbate is sodium salt of ascorbic acid (commonly known as vitamin C), which is approved for use as a food additive in many countries. Sodium ascorbate is consisted of a combination of sodium and vitamin C, which commonly serve as an antioxidant and an acidity regulator in pharmaceutical manufacturing and in the food industry. In this mixture, sodium acts as a buffer, creating a less acidic supplement than those made entirely from vitamin C. It can be easier to tolerate if the digestive system is sensitive to acid. As a vitamin C supplement, it provides both sodium and vitamin C for human body, which is effective to prevent or treat vitamin C deficiency. Besides, studies have shown that taking sodium ascorbate is helpful with cancer prevention and treatment.
References
https://en.wikipedia.org/wiki/Sodium_ascorbate
http://www.livestrong.com/article/348175-what-are-the-health-benefits-of-sodium-ascorbate/
http://pets.webmd.com/drugs/2/drug-63684/ascorbic-acid-ascorbate-sodium-oral/details
Chemical Properties
Sodium ascorbate occurs as a white or slightly yellow-colored,
practically odorless, crystalline powder with a pleasant saline taste.
Uses
Sodium Ascorbate is an antioxidant that is the sodium form of
ascorbic acid. it is soluble in water and provides a nonacidic taste. a
10% aqueous solution has a ph of 7.3–7.6. in water, it readily reacts
with atmospheric oxygen and other oxidizing agents, making it
valuable as an antioxidant. one part sodium ascorbate is equivalent
to 1.09 parts of sodium erythorbate. see ascorbic acid.
Uses
As antimicrobial and antioxidant in foodstuffs.
Uses
L-Ascorbic acid (Vitamin C) is a water soluble molecule used in a wide variety of applications, including cell culture, as a reducing agent that helps reduce oxidative stress.
Production Methods
An equivalent amount of sodium bicarbonate is added to a solution
of ascorbic acid in water. Following the cessation of effervescence,
the addition of propan-2-ol precipitates sodium ascorbate.
Definition
ChEBI: Sodium ascorbate is an organic sodium salt resulting from the replacement of the proton from the 3-hydroxy group of ascorbic acid by a sodium ion. It has a role as a food antioxidant, a flour treatment agent, a coenzyme, a plant metabolite, a human metabolite, a Daphnia magna metabolite and a reducing agent. It is an organic sodium salt and a vitamin C. It contains a L-ascorbate.
brand name
Ascorbin (Marion Merrell Dow).
General Description
Minute crystals or white powder. pH of aqueous solutions 5.6 to 7.0 or even higher (a 10% solution, made from a commercial grade, may have a pH of 7.4 to 7.7).
Air & Water Reactions
Water soluble. Aqueous solutions are subject to quick air oxidation at pH greater than 6.0.
Reactivity Profile
A weak base. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
SYMPTOMS: Ingestion of 10 grams or more of this type of compound may cause diarrhea.
Fire Hazard
Flash point data for Sodium ascorbate are not available. Sodium ascorbate is probably combustible.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Sodium ascorbate is used as an antioxidant in pharmaceutical
formulations, and also in food products where it increases the
effectiveness of sodium nitrite against growth of Listeria monocytogenes
in cooked meats. It improves gel cohesiveness and sensory
firmness of fiberized products regardless of vacuum treatment.
It is also used therapeutically as a source of vitamin C in tablets
and parenteral preparations.
Biochem/physiol Actions
Ascorbic acid exhibits anti-oxidant properties. It is a primary substrate for detoxifying hydrogen peroxide. Ascorbic acid is a co-factor for the synthesis of adrenal steroids and catecholamines. L-Ascorbic acid (Vitamin C) is a water soluble molecule used in a wide variety of applications, including cell culture, as a reducing agent that helps reduce oxidative stress. L-Ascorbate can be regenerated by biological systems.
Safety Profile
Human mutation data
reported. When heated to decomposition it
emits toxic fumes of Na2O.
Safety
The parenteral administration of 0.25-1.00 g of sodium ascorbate,
given daily in divided doses, is recommended in the treatment of
vitamin C deficiencies. Various adverse reactions have been
reported following the administration of 1 g or more of sodium
ascorbate, although ascorbic acid and sodium ascorbate are usually
well tolerated; see Ascorbic acid. There have been no reports of
adverse effects associated with the much lower concentrations of
sodium ascorbate and ascorbic acid, which are employed as
antioxidants.
The WHO has set an acceptable daily intake of ascorbic acid,
potassium ascorbate, and sodium ascorbate, as antioxidants in
food, at up to 15 mg/kg body-weight in addition to that naturally
present in food.
storage
Sodium ascorbate is relatively stable in air, although it gradually
darkens on exposure to light. Aqueous solutions are unstable and
subject to rapid oxidation in air at pH > 6.0.
The bulk material should be stored in a well-closed nonmetallic
container, protected from light, in a cool, dry place.
Incompatibilities
Incompatible with oxidizing agents, heavy metal ions, especially
copper and iron, methenamine, sodium nitrite, sodium salicylate,
and theobromine salicylate. The aqueous solution is reported to be
incompatible with stainless steel filters.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (IV preparations;
oral tablets). Included in nonparenteral and parenteral
medicines licensed in the UK. Included in the Canadian List of
Acceptable Non-medicinal Ingredients.