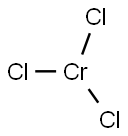
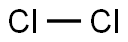Description
Chromic chloride is an odorless, purple or violet, flake-like orcrystalline (sand-like) solid.

Uses
Chromic chloride is used in chrome plating, as acatalyst, textile mordant, and corrosion inhibitor, and to make other chromium compounds.
Preparation
Chromic chloride may be prepared by chlorination of chromium metal directly, or indirectly by carbothermic chlorination of chromium(III) oxide at 650–800 °C:
Cr2O3 + 3 C + 3 Cl2 → 2 CrCl3 + 3 CO
Mechanism of action
Metallic chromium has no biological activity. Chromium is referred as a glucose tolerance factor. This glucose tolerance factor is a complex of molecules. Glycine, cysteine, glutamic acid and nicotinic acid along with chromium form this complex.
Structure and conformation
Anhydrous chromium(III) chloride adopts the YCl3 structure, with Cr3+ occupying one third of the octahedral interstices in alternating layers of a pseudo-cubic close packed lattice of Cl− ions. The absence of cations in alternate layers leads to weak bonding between adjacent layers.
Toxicity
Reported toxic reactions to chromium include nausea, vomiting, ulcers of the gastrointestinal tract, renal and hepatic damage, convulsions and coma. The acute LD50 for intravenous trivalent chromium in rats was reported as 10 to 18 mg/kg.
Incompatibilities
Reacts with water and strong oxidizers. Contact with strong acids or acid fumes may produce highly toxic chloride fumes. May attack certain steels, causing pitting attack and stress corrosion