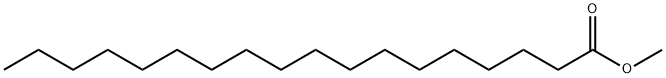
Chemical Properties
off-white to cream or light yellow paste
Uses
Methyl stearate is used as a nonionic surfactant, thereby enhancing the solubility of chemicals by dissociating aggregates and unfolding proteins. It is a fatty acid ester, which is used as an emulsifier and stabilizer.
Uses
Methyl Stearate is used as a nonionic surfactant in various experiments in helping solubilize a variety of chemical species by dissociating aggregates and unfolding proteins.
Definition
ChEBI: A natural product found in Neolitsea daibuensis.
Synthesis Reference(s)
Canadian Journal of Chemistry, 59, p. 2617, 1981
DOI: 10.1139/v81-377
General Description
White crystals or chunky solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
METHYL STEARATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition METHYL STEARATE emits toxic fumes.
Fire Hazard
METHYL STEARATE is combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Questionable
carcinogen with experimental tumorigenic
data. Combustible when exposed to heat or
flame; can react with oxidizing materials. To
fight fire, use CO2, dry chemical. When
heated to decomposition it emits acrid
smoke and irritating fumes.