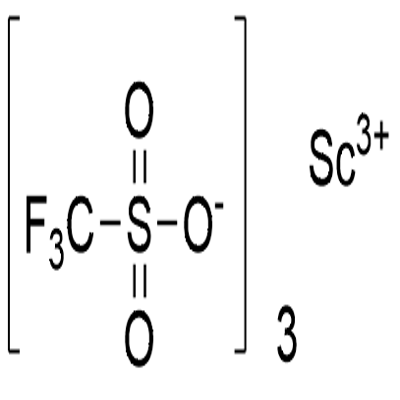
Description
Scandium triflate, commonly called Scandium trifluoromethanesulfonate, is a chemical compound with the formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3- anions. It is commercially available and is a practical and useful Lewis acid catalyst for the acylation of alcohols with acid anhydrides or the esterification of alcohols by carboxylic acids in the presence of p-nitrobenzoic anhydrides.
Uses
Scandium Triflate is widely used as a catalyst in hydrothiolation, selective two-electron reduction of oxygen by ferrocene derivatives and vinylogous Fridel-crafts alkylation of indoles and pyrrole in water. It is involved in the Mukaiyama aldol addition and stereochemically catalyzes the radical polymerization of acrylates. It was used as a catalyst in:
Hydrothiolation reaction of aromatic and aliphatic thiols.
Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
Synthesis of β-cyanoketones.
Combination with triethylsilane to reductively open functionalized pyranoside rings.
The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Preparation
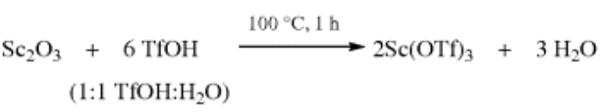
Scandium triflate is commercially available. On the other hand, it can also be prepared from the corresponding oxide (Sc2O3) and aqueous trifluoromethanesulfonic acid (TfOH). After filtration and concentration of the clear aqueous solution in vacuo, the resulting hydrated salt is dried in vacuo (<1 mmHg) at 200 °C for 40 h to afford the anhydrous triflate, which is stored over P2O5.
Solubility in water
Soluble in H2O, alcohol, acetonitrile, and most polar organic solvents.