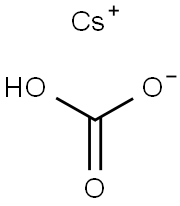
Physical properties
In cesium hydrogen carbonate, the cesium ion and the hydrogencarbonate anion are held together by ionic bonding. This type of bonding, which involves the electrostatic attraction between positively and negatively charged ions, is typically strong and results in the formation of a solid crystalline material at room temperature.
Uses
Cesium hydrogen carbonate is used as a source of bicarbonate ions.It can also be used as a catalyst for the:
Etherification of glycerol to oligomers.
Acetylation of primary alcohols and phenols to acetates using acetic anhydride.