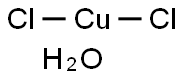Physical properties
Copper Chloride Dihydrate appears as a green crystalline powder with no odor and occurs naturally as the mineral eriochalcite. It is soluble in Water, Alcohol, and Acetone at ambient conditions.
Uses
Cupric Chloride Dihydrate is industrially produced for use as a co-catalyst in the Wacker process.
Preparation
Cupric chloride dihydrate can be produced either by the reaction of copper with chlorine or by combining copper carbonate with Hydrochloric Acid.
Chemical Reactivity
When heated to decomposition, cupric chloride dihydrate emits toxic fumes of hydrogen chloride gas and copper oxides.
Structure and conformation
In Cupric Chloride Dihydrate, the copper again adopts a highly distorted octahedral geometry, the Cu(II) centers being surrounded by two water ligands and four chloride ligands, which bridge asymmetrically to other Cu centers.
Toxicity
Cupric Chloride Dihydrate can be toxic. If copper chloride is absorbed, it results in headache, diarrhea, a drop in blood pressure, and fever. Ingestion of large amounts may induce copper poisoning, CNS disorders, and haemolysis.