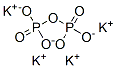
Chemical Properties
Tetrapotassium pyrophosphate is a white crystalline powder or granules and has a strong moisture absorption capability in air and is easily soluble in water but insoluble in ethanol. Its aqueous solution is alkaline and has effect on inhibition of food spoilage and fermentation.

Uses
Tetrapotassium pyrophosphate is mostly used in combination with other condensed phosphates. It is commonly used for preventing the generation of struvite in canned fish, preventing the discoloration of canned fruit, increasing the swelling degree of ice cream, the extracting amount of the raw materials of coffee as well as the yield of ham and sausage.
Preparation
Tetrapotassium pyrophosphate is produced by the reaction of furnace-grade phosphoric acid with sodium carbonate to form disodium phosphate, which is then heated to 450 °C to form Tetrapotassium pyrophosphate:
2 Na2HPO4 → Na4P2O7 + H2O
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Toxicity evaluation
ADI 0~70mg/k (the total amount of phosphorus calculated from phosphate; FAO/WHO, 2001).
It may cause kidney stones in large doses.
Adl 0~70 mg/kg (in terms of phosphorus); According to the provision of the FAO/WHO (1984): for the processed cheese, the total phosphate is 9 g/kg (in terms of phosphorus); luncheon meat: 3 g/kg; frozen shrimp or prawns: 5 g/kg; cooked meat 3 g/kg (based on anhydrous matter).