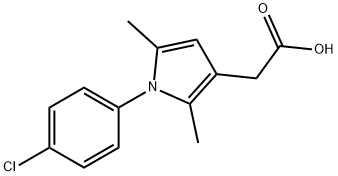
Clopirac
Bezeichnung:Clopirac
CAS-Nr42779-82-8
Englisch Name:1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci
CBNumberCB9869925
SummenformelC14H14ClNO2
Molgewicht263.72
MOL-Datei42779-82-8.mol
Synonyma
Clopirac
Clopirac physikalisch-chemischer Eigenschaften
| Siedepunkt | 429.1±45.0 °C(Predicted) |
| Dichte | 1.23±0.1 g/cm3(Predicted) |
| pka | 4.38±0.10(Predicted) |
