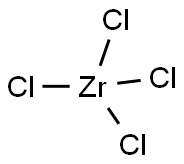
Zirconiumchlorid
Bezeichnung:Zirconiumchlorid
CAS-Nr10026-11-6
Englisch Name:Zirconium tetrachloride
CBNumberCB8298375
SummenformelCl4Zr
Molgewicht233.04
MOL-Datei10026-11-6.mol
Synonyma
Zirconiumtetrachlorid
Zirconiumchlorid
Zirconiumchlorid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 437 °C | ||||||||||||||
| Siedepunkt | 331°C | ||||||||||||||
| Dichte | 2.8 g/mL at 25 °C(lit.) | ||||||||||||||
| chüttdichte | 1200kg/m3 | ||||||||||||||
| Dampfdruck | 1 mm Hg ( 190 °C) | ||||||||||||||
| storage temp. | Store below +30°C. | ||||||||||||||
| Löslichkeit | Soluble in alcohol, ether, hydrochloric acid. | ||||||||||||||
| Aggregatzustand | powder | ||||||||||||||
| Farbe | White | ||||||||||||||
| Wichte | 2.803 | ||||||||||||||
| PH | <1 (H2O, 20℃)(saturated aqueous solution) | ||||||||||||||
| Wasserlöslichkeit | Reacts with water. | ||||||||||||||
| Sensitive | Moisture Sensitive & Hygroscopic | ||||||||||||||
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | ||||||||||||||
| Merck | 14,10174 | ||||||||||||||
| crystal system | Monoclinic | ||||||||||||||
| Space group | P2/c | ||||||||||||||
| Lattice constant |
|
| Kennzeichnung gefährlicher | C |
| R-Sätze: | 14-22-34-35 |
| S-Sätze: | 26-36/37/39-45-8 |
| RIDADR | UN 2503 8/PG 3 |
| WGK Germany | 3 |
| RTECS-Nr. | ZH7175000 |
| F | 19 |
| TSCA | Yes |
| HS Code | 2827 39 85 |
| HazardClass | 8 |
| PackingGroup | III |
| Giftige Stoffe Daten | 10026-11-6(Hazardous Substances Data) |
| Toxizität | LD50 in mice, rats (mg/kg): 665, 1688 orally (Zhilova, Kasparov) |

